Mastering Electron Configuration Worksheets
Understanding Electron Configuration
Electron configuration is a crucial concept in chemistry that describes the arrangement of electrons in an atom. It is a fundamental principle in understanding the chemical properties and behavior of elements. Mastering electron configuration worksheets is essential for students to grasp this concept and apply it to various chemical reactions and processes.
What is Electron Configuration?
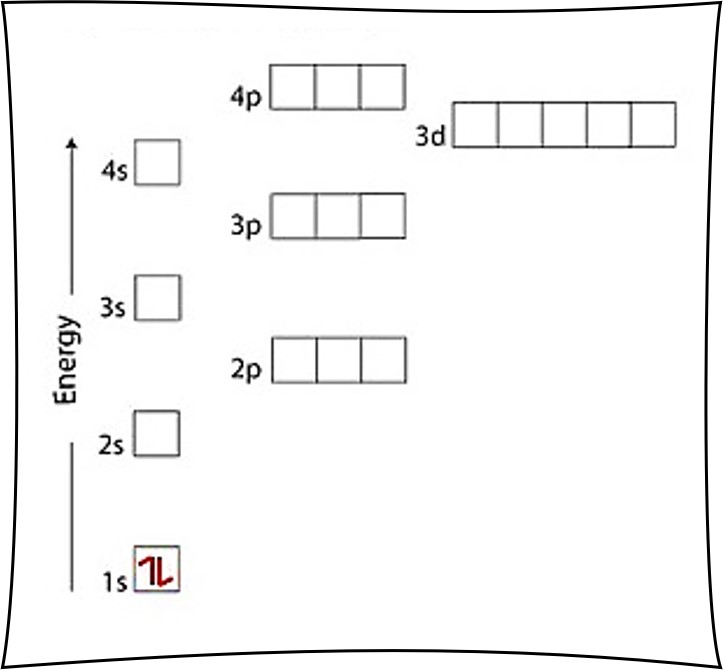
Electron configuration is the arrangement of electrons in an atom, which is determined by the number of protons in the nucleus. The electrons are arranged in energy levels or shells, with each shell having a specific capacity. The electrons in each shell are further divided into subshells, which are designated by the letters s, p, d, and f.
How to Write Electron Configuration
Writing electron configuration involves determining the number of electrons in each energy level and subshell. The electrons are arranged in the following order:
- s subshell: 2 electrons
- p subshell: 6 electrons
- d subshell: 10 electrons
- f subshell: 14 electrons
The electron configuration is written in a specific notation, which includes the energy level, subshell, and number of electrons. For example, the electron configuration of hydrogen is 1s1, indicating that there is one electron in the s subshell of the first energy level.
Steps to Write Electron Configuration
- Determine the number of electrons: The first step is to determine the number of electrons in the atom. This can be done by looking at the atomic number of the element.
- Determine the energy levels: The next step is to determine the number of energy levels in the atom. This can be done by looking at the periodic table.
- Determine the subshells: The next step is to determine the number of subshells in each energy level.
- Arrange the electrons: The final step is to arrange the electrons in the subshells, following the Aufbau principle and the Pauli Exclusion Principle.
🤔 Note: The Aufbau principle states that electrons occupy the lowest available energy levels, while the Pauli Exclusion Principle states that each subshell can hold a maximum of two electrons with opposite spins.
Types of Electron Configuration
There are two types of electron configuration: ground-state electron configuration and excited-state electron configuration.
- Ground-state electron configuration: This is the most stable arrangement of electrons in an atom.
- Excited-state electron configuration: This is a temporary arrangement of electrons that occurs when an electron is excited to a higher energy level.
Electron Configuration Worksheets
Mastering electron configuration worksheets involves practicing the steps to write electron configuration. Here are some tips to help you master electron configuration worksheets:
- Practice, practice, practice: The more you practice, the more comfortable you will become with writing electron configuration.
- Use the periodic table: The periodic table is a valuable resource for determining the number of electrons and energy levels in an atom.
- Check your work: Always check your work to ensure that you have correctly written the electron configuration.
Common Mistakes to Avoid
Here are some common mistakes to avoid when working on electron configuration worksheets:
- Incorrect number of electrons: Make sure to accurately determine the number of electrons in the atom.
- Incorrect energy levels: Make sure to accurately determine the number of energy levels in the atom.
- Incorrect subshells: Make sure to accurately determine the number of subshells in each energy level.
🤔 Note: Double-check your work to avoid these common mistakes.
Conclusion
Mastering electron configuration worksheets requires practice and attention to detail. By following the steps to write electron configuration and avoiding common mistakes, you can become proficient in writing electron configuration. Remember to always check your work and use the periodic table as a resource.
What is the Aufbau principle?
+
The Aufbau principle states that electrons occupy the lowest available energy levels.
What is the Pauli Exclusion Principle?
+
The Pauli Exclusion Principle states that each subshell can hold a maximum of two electrons with opposite spins.
How many electrons can the s subshell hold?
+
The s subshell can hold 2 electrons.
Related Terms:
- Electron configuration pdf