Mastering Chemical Formulas with the Criss Cross Method
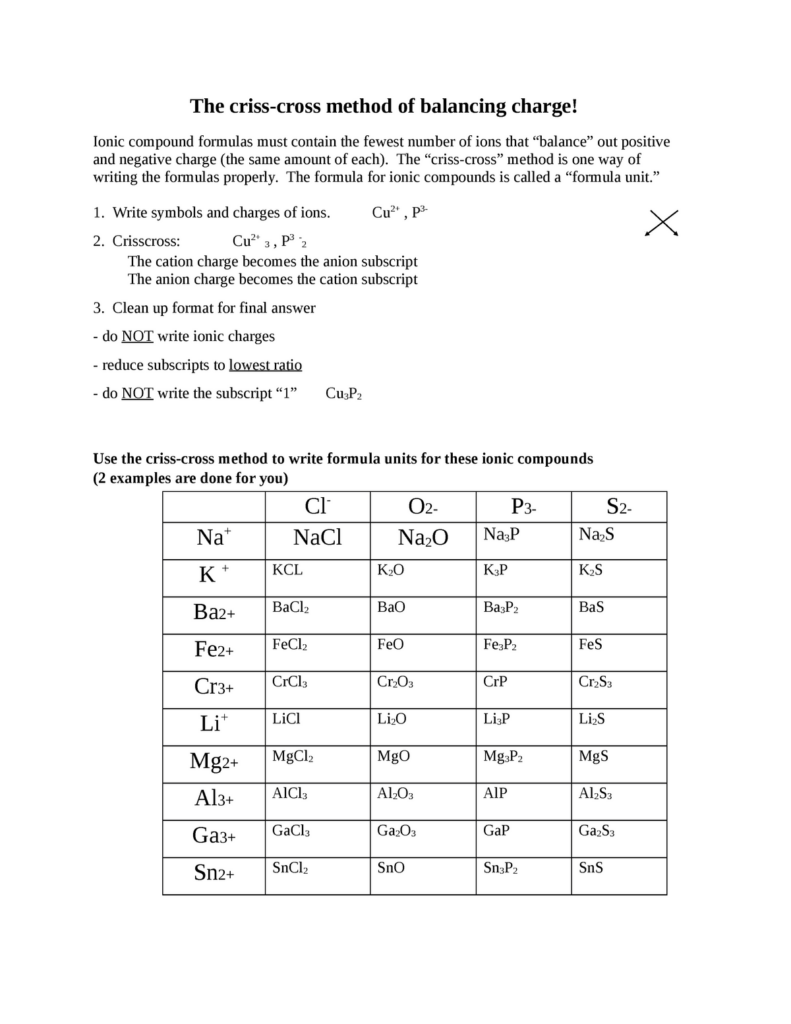
Unlocking the Power of Chemical Formulas with the Criss Cross Method
Chemical formulas can be intimidating, especially for those new to chemistry. However, with the right approach, mastering chemical formulas can become a breeze. One of the most effective methods for balancing chemical equations and writing formulas is the Criss Cross Method. In this article, we will delve into the world of chemical formulas and explore how the Criss Cross Method can help you become a master of chemical equations.
Understanding Chemical Formulas
Before we dive into the Criss Cross Method, it’s essential to understand the basics of chemical formulas. A chemical formula is a way of expressing the composition of a molecule using chemical symbols. It shows the number of atoms of each element present in the molecule. For example, the formula for water is H2O, indicating that one molecule of water consists of two hydrogen atoms and one oxygen atom.
The Criss Cross Method: A Step-by-Step Guide
The Criss Cross Method is a simple and effective technique for balancing chemical equations and writing formulas. Here’s a step-by-step guide to get you started:
- Step 1: Write the unbalanced equation. Start by writing the unbalanced chemical equation, using the chemical symbols for each element.
- Step 2: Count the atoms. Count the number of atoms of each element on both the reactant and product sides of the equation.
- Step 3: Identify the imbalanced elements. Identify the elements that have a different number of atoms on the reactant and product sides.
- Step 4: Apply the Criss Cross Method. To balance the equation, use the Criss Cross Method. This involves:
- Criss: Crossing the number of atoms of an element from the reactant side to the product side.
- Cross: Crossing the number of atoms of an element from the product side to the reactant side.
- Step 5: Balance the equation. Continue applying the Criss Cross Method until the equation is balanced.
Example: Balancing a Chemical Equation with the Criss Cross Method
Let’s use the Criss Cross Method to balance the following equation:
Na + O2 → Na2O
Step 1: Write the unbalanced equation
Na + O2 → Na2O
Step 2: Count the atoms
| Element | Reactant Side | Product Side |
|---|---|---|
| Na | 1 | 2 |
| O | 2 | 1 |
Step 3: Identify the imbalanced elements
The elements Na and O are imbalanced.
Step 4: Apply the Criss Cross Method
- Criss: Cross the number of Na atoms from the reactant side to the product side (1 → 2).
- Cross: Cross the number of O atoms from the product side to the reactant side (1 → 2).
Step 5: Balance the equation
The balanced equation is:
2Na + O2 → 2Na2O
However, this is not the final answer. We need to ensure that the equation is balanced in terms of both atoms and charges.
Final answer:
4Na + O2 → 2Na2O
Advantages of the Criss Cross Method
The Criss Cross Method offers several advantages, including:
- Easy to learn: The Criss Cross Method is a simple and intuitive technique that can be learned quickly.
- Effective: The Criss Cross Method is an effective technique for balancing chemical equations and writing formulas.
- Reduced errors: The Criss Cross Method minimizes errors, as it ensures that the equation is balanced in terms of both atoms and charges.
📝 Note: The Criss Cross Method is a useful technique for balancing simple chemical equations. However, it may not be effective for complex equations or equations with multiple reactants and products.
Common Mistakes to Avoid
When using the Criss Cross Method, it’s essential to avoid common mistakes, including:
- Insufficient counting: Failing to count the atoms of each element on both the reactant and product sides.
- Incorrect application: Applying the Criss Cross Method incorrectly, resulting in an unbalanced equation.
Conclusion
Mastering chemical formulas with the Criss Cross Method requires practice and patience. By following the steps outlined in this article, you can become proficient in using the Criss Cross Method to balance chemical equations and write formulas. Remember to avoid common mistakes and use the Criss Cross Method in conjunction with other techniques to ensure accuracy.
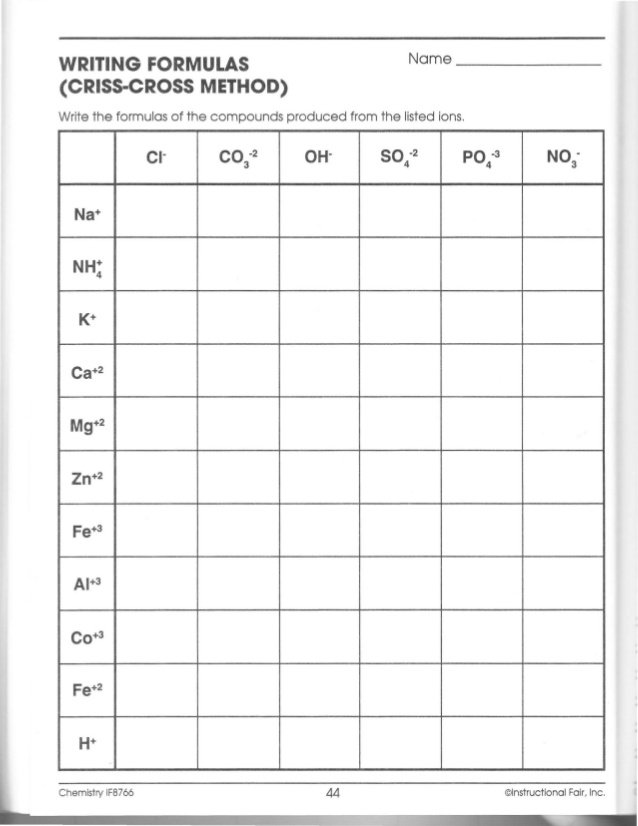
What is the Criss Cross Method?
+The Criss Cross Method is a technique used to balance chemical equations and write formulas. It involves crossing the number of atoms of an element from the reactant side to the product side and vice versa.
What are the advantages of the Criss Cross Method?
+The Criss Cross Method is easy to learn, effective, and reduces errors. It’s a useful technique for balancing simple chemical equations.
What are common mistakes to avoid when using the Criss Cross Method?
+Common mistakes to avoid include insufficient counting and incorrect application of the Criss Cross Method. It’s essential to count the atoms of each element on both the reactant and product sides and apply the Criss Cross Method correctly.
Related Terms:
- Criss cross method in chemistry
- criss-cross method examples
- criss-cross method worksheet pdf
- Criss-cross method Practice
- Sodium chloride criss cross method



