5 Essential Facts About Isotopes You Must Know
Unlocking the Secrets of Isotopes: A Comprehensive Guide
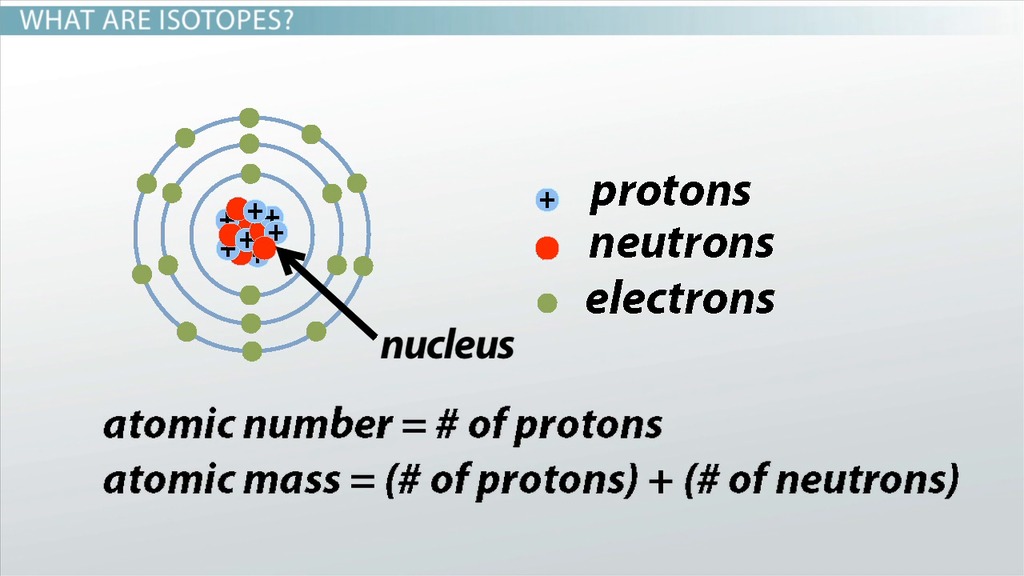
Isotopes are a fundamental concept in chemistry and physics, yet many people are unaware of their significance and importance. Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This difference in neutron number leads to variations in the physical and chemical properties of isotopes. In this article, we will delve into the world of isotopes and explore five essential facts that you must know.
Fact #1: Isotopes are Used in Medicine
Isotopes have numerous applications in medicine, particularly in the field of nuclear medicine. Radioactive isotopes are used to diagnose and treat various diseases, including cancer. For example, technetium-99m is a radioactive isotope used in imaging procedures to diagnose a range of conditions, from cancer to heart disease. Additionally, iodine-131 is used to treat thyroid cancer.
💡 Note: Radioactive isotopes can be both beneficial and hazardous, so handling and disposal require extreme care.
Fact #2: Isotopes Play a Crucial Role in Food Safety
Isotopes are used to detect and prevent food adulteration. Stable isotope analysis is a technique used to identify the origin and authenticity of food products. This method involves analyzing the isotope ratios of elements such as carbon, nitrogen, and oxygen in food samples. By comparing these ratios to known standards, scientists can determine the food’s origin and detect any tampering or adulteration.
Fact #3: Isotopes Help Us Understand Climate Change
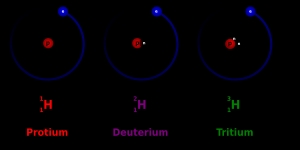
Isotopes are essential tools in the study of climate change. Oxygen-18 and deuterium are two isotopes used to analyze the Earth’s climate history. By studying the isotope ratios in ice cores and sediment samples, scientists can reconstruct past climate conditions and gain insights into the Earth’s climate system. This information helps researchers understand the impact of human activities on the climate and develop more accurate climate models.
Fact #4: Isotopes are Used in Archaeology
Isotopes are used in archaeology to date artifacts and reconstruct the past. Carbon-14 is a radioactive isotope used to determine the age of organic materials, such as wood and bone. By analyzing the amount of carbon-14 in an artifact, archaeologists can estimate its age and gain insights into the past. Additionally, strontium isotopes are used to analyze the origins of artifacts and reconstruct ancient trade networks.
Fact #5: Isotopes Have Industrial Applications
Isotopes have numerous industrial applications, including the production of semiconductors and laser technology. Silicon-28 and silicon-30 are two isotopes used to produce high-purity silicon, a critical component in the manufacture of semiconductors. Additionally, neodymium isotopes are used in the production of high-powered lasers.
| Isotope | Application |
|---|---|
| Technetium-99m | Nuclear medicine |
| Iodine-131 | Cancer treatment |
| Oxygen-18 | Climate research |
| Carbon-14 | Archaeological dating |
| Silicon-28 | Semiconductor production |
In conclusion, isotopes play a vital role in various fields, from medicine and food safety to climate research and industrial applications. By understanding the properties and applications of isotopes, we can unlock new technologies and improve our daily lives. Whether it’s detecting food adulteration or treating cancer, isotopes are an essential tool in modern science.
What is the difference between a radioactive isotope and a stable isotope?
+A radioactive isotope is an isotope that decays into another element, emitting radiation in the process. A stable isotope, on the other hand, is an isotope that does not undergo radioactive decay.
How are isotopes used in medicine?
+Isotopes are used in medicine for diagnostic and therapeutic purposes. Radioactive isotopes are used to diagnose diseases, while stable isotopes are used to treat conditions such as cancer.
What is the most common application of isotopes?
+The most common application of isotopes is in the field of medicine, particularly in nuclear medicine.
Related Terms:
- Isotope Worksheet pdf
- Isotope Practice Worksheet answers PDF
- Isotope Practice Worksheet Answer Key
- Isotopes worksheet doc
- Chemistry isotopes Worksheet