Mastering Mole Conversions: A Chemistry Worksheet Guide

Introduction to Mole Conversions
Mole conversions are a fundamental concept in chemistry, and mastering them is essential for any student or professional in the field. In this guide, we will walk you through the basics of mole conversions, provide a step-by-step tutorial on how to perform them, and offer some tips and tricks to help you become a pro.
What is a Mole?
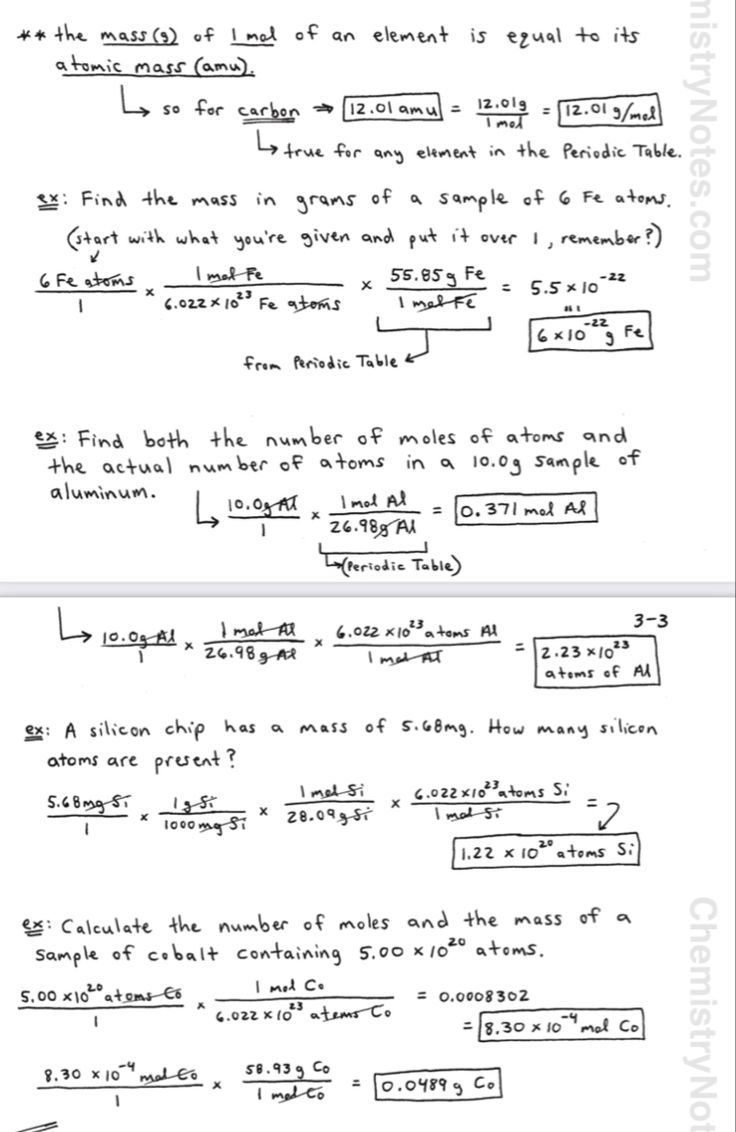
A mole (mol) is the unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains as many particles (atoms, molecules, or ions) as there are atoms in 0.012 kilograms of carbon-12. This number is known as the Avogadro’s constant (NA) and is approximately equal to 6.022 x 10^23 particles.
Why are Mole Conversions Important?
Mole conversions are crucial in chemistry because they allow us to convert between different units of measurement, such as grams to moles, moles to liters, and liters to grams. This is essential for calculating the amount of a substance required for a reaction, determining the concentration of a solution, and predicting the outcome of a chemical reaction.
Step-by-Step Guide to Mole Conversions
Here is a step-by-step guide to performing mole conversions:
Step 1: Identify the Given Information
Identify the given information, such as the mass of a substance in grams, the number of moles, or the volume of a solution in liters.
Step 2: Determine the Conversion Factor
Determine the conversion factor needed to perform the conversion. For example, if you want to convert grams to moles, the conversion factor is the molar mass of the substance.
Step 3: Set Up the Conversion
Set up the conversion by writing the given information and the conversion factor in a mathematical equation.
Step 4: Perform the Conversion
Perform the conversion by multiplying or dividing the given information by the conversion factor.
Step 5: Check Your Units
Check your units to ensure that they match the desired units.
Example 1: Converting Grams to Moles
Problem: Convert 25 grams of sodium chloride (NaCl) to moles.
Given information: 25 g NaCl Conversion factor: Molar mass of NaCl = 58.44 g/mol
Step 1: Identify the given information Step 2: Determine the conversion factor Step 3: Set up the conversion 25 g NaCl x (1 mol / 58.44 g) =? Step 4: Perform the conversion 25 g NaCl x (1 mol / 58.44 g) = 0.428 mol Step 5: Check your units Units are correct (moles)
Example 2: Converting Moles to Liters
Problem: Convert 2 moles of carbon dioxide (CO2) to liters at standard temperature and pressure (STP).
Given information: 2 mol CO2 Conversion factor: Molar volume of a gas at STP = 22.4 L/mol
Step 1: Identify the given information Step 2: Determine the conversion factor Step 3: Set up the conversion 2 mol CO2 x (22.4 L / 1 mol) =? Step 4: Perform the conversion 2 mol CO2 x (22.4 L / 1 mol) = 44.8 L Step 5: Check your units Units are correct (liters)
🤔 Note: Always check your units to ensure that they match the desired units.
Tips and Tricks
- Always use the correct conversion factor for the specific problem.
- Check your units to ensure that they match the desired units.
- Use dimensional analysis to ensure that the units cancel out correctly.
- Practice, practice, practice! The more you practice mole conversions, the more comfortable you will become.
Common Mole Conversion Factors
Here are some common mole conversion factors:
| Conversion Factor | Description |
|---|---|
| 1 mol = 6.022 x 10^23 particles | Avogadro’s constant |
| 1 mol = 22.4 L (at STP) | Molar volume of a gas at STP |
| 1 mol = 1 g/mol x molar mass | Conversion factor for grams to moles |
Conclusion
Mastering mole conversions is essential for any student or professional in chemistry. By following the step-by-step guide outlined in this tutorial, you can become proficient in performing mole conversions and tackle even the most challenging problems with confidence. Remember to always check your units and practice regularly to become a pro at mole conversions.
What is the definition of a mole?
+A mole (mol) is the unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains as many particles (atoms, molecules, or ions) as there are atoms in 0.012 kilograms of carbon-12.
Why are mole conversions important in chemistry?
+Mole conversions are crucial in chemistry because they allow us to convert between different units of measurement, such as grams to moles, moles to liters, and liters to grams. This is essential for calculating the amount of a substance required for a reaction, determining the concentration of a solution, and predicting the outcome of a chemical reaction.
What is the molar mass of a substance?
+The molar mass of a substance is the mass of one mole of that substance, typically expressed in units of grams per mole (g/mol).