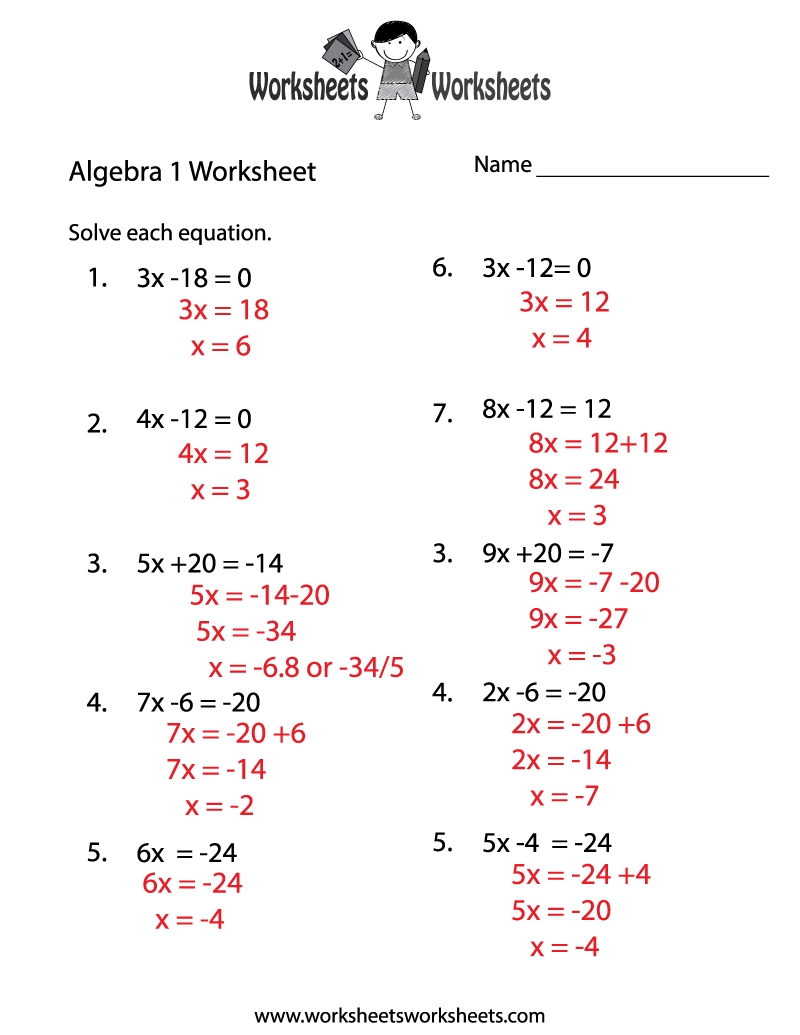
5 Essential Acids Bases and Salts Worksheet Tips

Understanding Acids, Bases, and Salts: A Comprehensive Guide
Acids, bases, and salts are fundamental concepts in chemistry, and understanding their properties and reactions is crucial for students and professionals alike. In this article, we will delve into the world of acids, bases, and salts, exploring their definitions, types, and characteristics. We will also provide essential tips and tricks for mastering acids, bases, and salts worksheets.
What are Acids, Bases, and Salts?
Before we dive into the tips and tricks, let’s first define what acids, bases, and salts are.
- Acids: Acids are substances that donate a hydrogen ion (H+), also known as a proton. They are typically sour in taste and turn blue litmus paper red.
- Bases: Bases are substances that accept a hydrogen ion (H+), also known as a proton. They are typically bitter in taste and turn red litmus paper blue.
- Salts: Salts are substances that are formed when an acid reacts with a base. They are typically neutral in taste and do not affect the color of litmus paper.
Types of Acids and Bases
There are several types of acids and bases, including:
- Strong Acids: Strong acids are acids that completely dissociate in water, releasing a high concentration of hydrogen ions. Examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
- Weak Acids: Weak acids are acids that partially dissociate in water, releasing a low concentration of hydrogen ions. Examples include acetic acid (CH3COOH) and citric acid (C6H8O7).
- Strong Bases: Strong bases are bases that completely dissociate in water, releasing a high concentration of hydroxide ions. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
- Weak Bases: Weak bases are bases that partially dissociate in water, releasing a low concentration of hydroxide ions. Examples include ammonia (NH3) and trimethylamine (N(CH3)3).
Essential Tips for Acids, Bases, and Salts Worksheets
Now that we have covered the basics, here are some essential tips for mastering acids, bases, and salts worksheets:
- Understand the pH Scale: The pH scale is a measure of the concentration of hydrogen ions in a solution. A pH of 7 is neutral, while a pH below 7 is acidic and a pH above 7 is basic.
- Know the Acid-Base Reactions: Acid-base reactions involve the transfer of a hydrogen ion from an acid to a base. This reaction is also known as neutralization.
- Identify the Type of Acid or Base: Identify whether the acid or base is strong or weak, as this will affect the reaction.
- Balance the Equation: Make sure to balance the equation by adding or removing reactants and products as necessary.
- Use the Arrhenius Definition: Use the Arrhenius definition to identify whether a substance is an acid or a base.
Worksheet Tips
Here are some additional tips for completing acids, bases, and salts worksheets:
- Read the Question Carefully: Read the question carefully and make sure you understand what is being asked.
- Use the Periodic Table: Use the periodic table to identify the elements and their properties.
- Check Your Work: Check your work by plugging in the answers to the equation.
- Use Online Resources: Use online resources, such as chemistry websites and tutorials, to help you understand the concepts.
💡 Note: Practice makes perfect! Make sure to practice completing acids, bases, and salts worksheets to become proficient in the subject.
Common Acids, Bases, and Salts Worksheet Questions
Here are some common acids, bases, and salts worksheet questions:
- What is the pH of a solution with a hydrogen ion concentration of 0.01 M?
- What is the type of acid-base reaction between hydrochloric acid and sodium hydroxide?
- What is the balanced equation for the reaction between sulfuric acid and potassium hydroxide?
Example Answers
Here are the answers to the above questions:
- What is the pH of a solution with a hydrogen ion concentration of 0.01 M? The pH of the solution is 2.
- What is the type of acid-base reaction between hydrochloric acid and sodium hydroxide? The reaction is a strong acid-strong base reaction.
- What is the balanced equation for the reaction between sulfuric acid and potassium hydroxide? The balanced equation is: H2SO4 + 2KOH → K2SO4 + 2H2O
What is the difference between a strong acid and a weak acid?
+A strong acid is an acid that completely dissociates in water, releasing a high concentration of hydrogen ions. A weak acid is an acid that partially dissociates in water, releasing a low concentration of hydrogen ions.
What is the pH scale?
+The pH scale is a measure of the concentration of hydrogen ions in a solution. A pH of 7 is neutral, while a pH below 7 is acidic and a pH above 7 is basic.
What is the Arrhenius definition of an acid?
+The Arrhenius definition of an acid is a substance that donates a hydrogen ion (H+) in a solution.
In summary, mastering acids, bases, and salts worksheets requires a strong understanding of the concepts and properties of acids, bases, and salts. By following the tips and tricks outlined in this article, you can improve your skills and become proficient in completing acids, bases, and salts worksheets. Remember to practice regularly and use online resources to help you understand the concepts.
Related Terms:
- Acids and bases Worksheet PDF