VSEPR Theory Worksheet: Shape Your Molecular Understanding
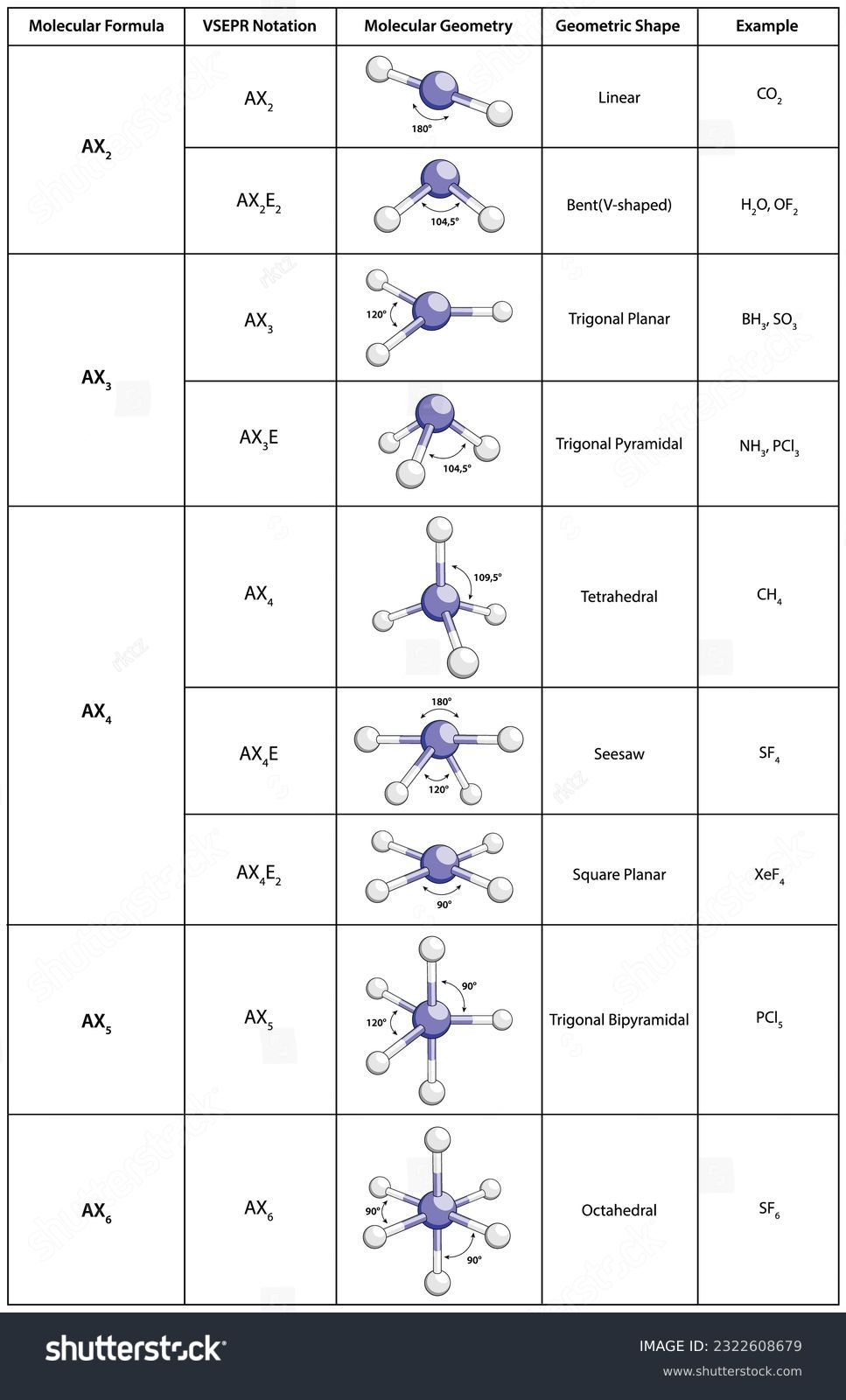
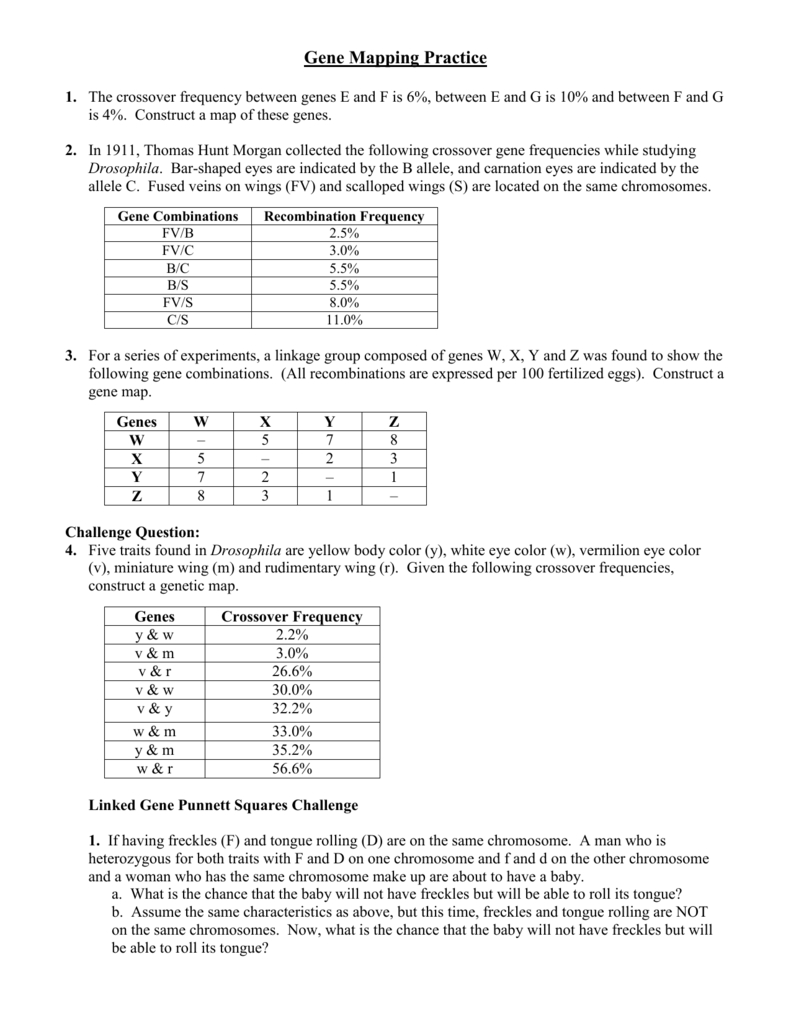
VSEPR Theory: A Fundamental Concept in Chemistry
VSEPR (Valence Shell Electron Pair Repulsion) theory is a fundamental concept in chemistry that helps us understand the shape of molecules. It is based on the idea that the electrons in the valence shell of an atom repel each other, resulting in a specific arrangement of atoms in a molecule. In this post, we will delve into the details of VSEPR theory, its applications, and provide a worksheet to help you practice and reinforce your understanding.
What is VSEPR Theory?
VSEPR theory was developed by Nevil Sidgwick and Herbert Powell in the 1940s. It states that the shape of a molecule is determined by the arrangement of its electron pairs in the valence shell. The theory assumes that electron pairs in the valence shell repel each other, resulting in a specific arrangement of atoms in a molecule.
Key Principles of VSEPR Theory
The following are the key principles of VSEPR theory:
- Electron pairs repel each other: Electron pairs in the valence shell of an atom repel each other, resulting in a specific arrangement of atoms in a molecule.
- Electron pairs occupy the most stable positions: Electron pairs occupy the most stable positions in the valence shell, which are determined by the number of electron pairs and the type of bond (single, double, or triple).
- Lone pairs occupy more space than bonded pairs: Lone pairs (non-bonding pairs) occupy more space than bonded pairs (bonding pairs), resulting in a greater repulsion between lone pairs.
Shapes of Molecules According to VSEPR Theory
According to VSEPR theory, the shape of a molecule is determined by the arrangement of its electron pairs in the valence shell. The following are some common shapes of molecules:

| Number of Electron Pairs | Shape |
|---|---|
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |
Worksheet: Applying VSEPR Theory
Here is a worksheet to help you practice and reinforce your understanding of VSEPR theory:
Question 1 What is the shape of a molecule with 3 electron pairs in the valence shell?
A) Linear B) Trigonal Planar C) Tetrahedral D) Octahedral
Answer: B) Trigonal Planar
Question 2 What is the shape of a molecule with 4 electron pairs in the valence shell, with 2 lone pairs?
A) Tetrahedral B) Trigonal Bipyramidal C) Octahedral D) Square Planar
Answer: A) Tetrahedral
Question 3 What is the shape of a molecule with 5 electron pairs in the valence shell, with 1 lone pair?
A) Trigonal Bipyramidal B) Octahedral C) Square Planar D) Tetrahedral
Answer: A) Trigonal Bipyramidal
Notes
📝 Note: VSEPR theory is a simplified model that assumes that electron pairs in the valence shell repel each other. However, it is a useful tool for predicting the shape of molecules and understanding the arrangement of atoms in a molecule.
Conclusion
VSEPR theory is a fundamental concept in chemistry that helps us understand the shape of molecules. By applying the principles of VSEPR theory, we can predict the shape of a molecule based on the arrangement of its electron pairs in the valence shell. With practice and reinforcement, you can master VSEPR theory and become proficient in predicting the shape of molecules.
What is the main principle of VSEPR theory?
+The main principle of VSEPR theory is that electron pairs in the valence shell of an atom repel each other, resulting in a specific arrangement of atoms in a molecule.
What is the shape of a molecule with 4 electron pairs in the valence shell?
+The shape of a molecule with 4 electron pairs in the valence shell is tetrahedral.
What is the difference between a lone pair and a bonded pair?
+A lone pair is a non-bonding pair, while a bonded pair is a pair of electrons involved in a chemical bond.
Related Terms:
- VSEPR theory Worksheet pdf
- VSEPR worksheet with answers pdf
- VSEPR theory Worksheet with answers
- Molecular geometry and VSEPR Worksheet
- VSEPR Worksheet high school pdf
- VSEPR and hybridization Worksheet