Type Of Reactions Worksheet Answers
Chemical reactions are the processes by which atoms or groups of atoms are transformed into other substances. There are several types of reactions, each with its own unique characteristics. Understanding the different types of reactions is crucial in chemistry and is a fundamental concept in various fields, including biology, physics, and engineering.
Types of Chemical Reactions
Chemical reactions can be classified into several types based on the nature of the reaction, the reactants, and the products formed. Here are some of the main types of chemical reactions:
1. Synthesis Reactions
- Definition: A synthesis reaction is a type of reaction where two or more reactants combine to form a new product.
- Example: 2H2 + O2 → 2H2O (hydrogen gas and oxygen gas combine to form water)
- Key Characteristic: Two or more reactants form a new product.
2. Decomposition Reactions
- Definition: A decomposition reaction is a type of reaction where a single reactant breaks down into two or more products.
- Example: 2H2O → 2H2 + O2 (water decomposes into hydrogen gas and oxygen gas)
- Key Characteristic: A single reactant breaks down into two or more products.
3. Replacement Reactions
- Definition: A replacement reaction, also known as a substitution reaction, is a type of reaction where one element or group of elements replaces another element or group of elements in a compound.
- Example: Zn + CuSO4 → ZnSO4 + Cu (zinc replaces copper in copper sulfate)
- Key Characteristic: One element or group of elements replaces another element or group of elements.
4. Combustion Reactions
- Definition: A combustion reaction is a type of reaction where a substance reacts with oxygen to produce heat and light.
- Example: CH4 + 2O2 → CO2 + 2H2O (methane reacts with oxygen to produce carbon dioxide and water)
- Key Characteristic: A substance reacts with oxygen to produce heat and light.
5. Neutralization Reactions
- Definition: A neutralization reaction is a type of reaction where an acid and a base react to form a salt and water.
- Example: HCl + NaOH → NaCl + H2O (hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water)
- Key Characteristic: An acid and a base react to form a salt and water.
Worksheet Answers
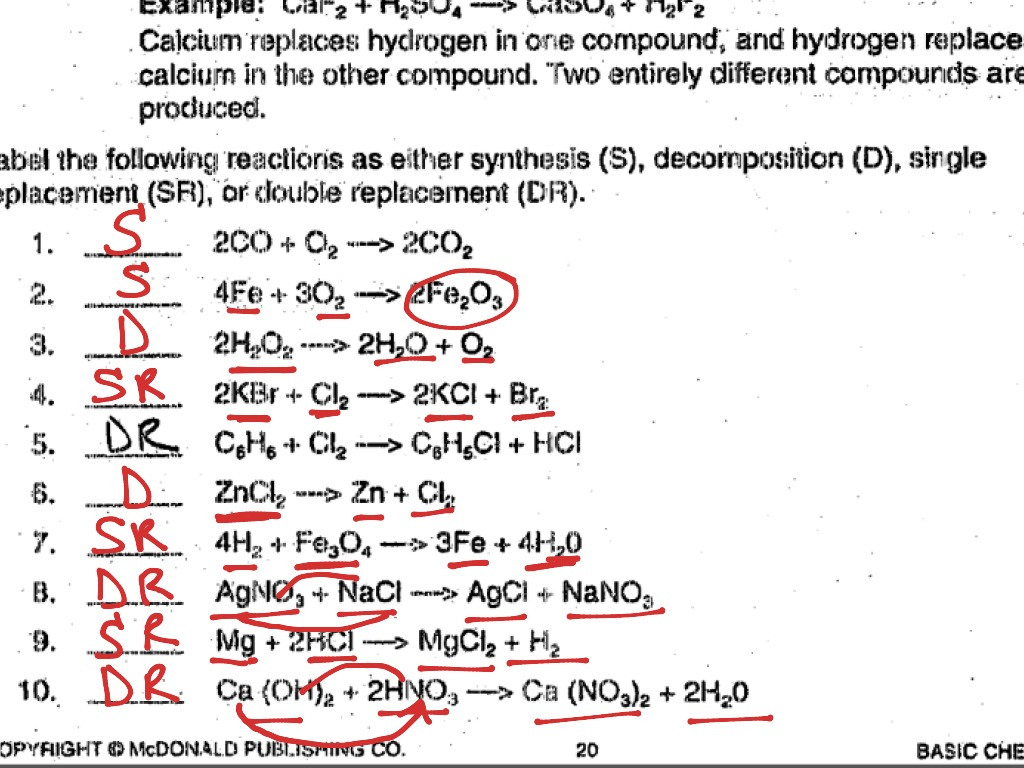
Here are the answers to a worksheet on the types of chemical reactions:
1. What type of reaction is 2H2 + O2 → 2H2O? Answer: Synthesis reaction
2. What type of reaction is 2H2O → 2H2 + O2? Answer: Decomposition reaction
3. What type of reaction is Zn + CuSO4 → ZnSO4 + Cu? Answer: Replacement reaction
4. What type of reaction is CH4 + 2O2 → CO2 + 2H2O? Answer: Combustion reaction
5. What type of reaction is HCl + NaOH → NaCl + H2O? Answer: Neutralization reaction
6. What is the key characteristic of a synthesis reaction? Answer: Two or more reactants combine to form a new product.
7. What is the key characteristic of a decomposition reaction? Answer: A single reactant breaks down into two or more products.
8. What is the key characteristic of a replacement reaction? Answer: One element or group of elements replaces another element or group of elements.
9. What is the key characteristic of a combustion reaction? Answer: A substance reacts with oxygen to produce heat and light.
10. What is the key characteristic of a neutralization reaction? Answer: An acid and a base react to form a salt and water.
📝 Note: This is a sample worksheet and answers. You can modify the questions and answers to fit your specific needs.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction involves the combination of two or more reactants to form a new product, while a decomposition reaction involves the breakdown of a single reactant into two or more products.
What is the key characteristic of a replacement reaction?
+The key characteristic of a replacement reaction is that one element or group of elements replaces another element or group of elements in a compound.
What is the key characteristic of a combustion reaction?
+The key characteristic of a combustion reaction is that a substance reacts with oxygen to produce heat and light.
In summary, understanding the different types of chemical reactions is crucial in chemistry and is a fundamental concept in various fields. By recognizing the key characteristics of each type of reaction, you can better understand the processes involved and apply this knowledge to solve problems and answer questions.