5 Essential Facts on Atomic Structure

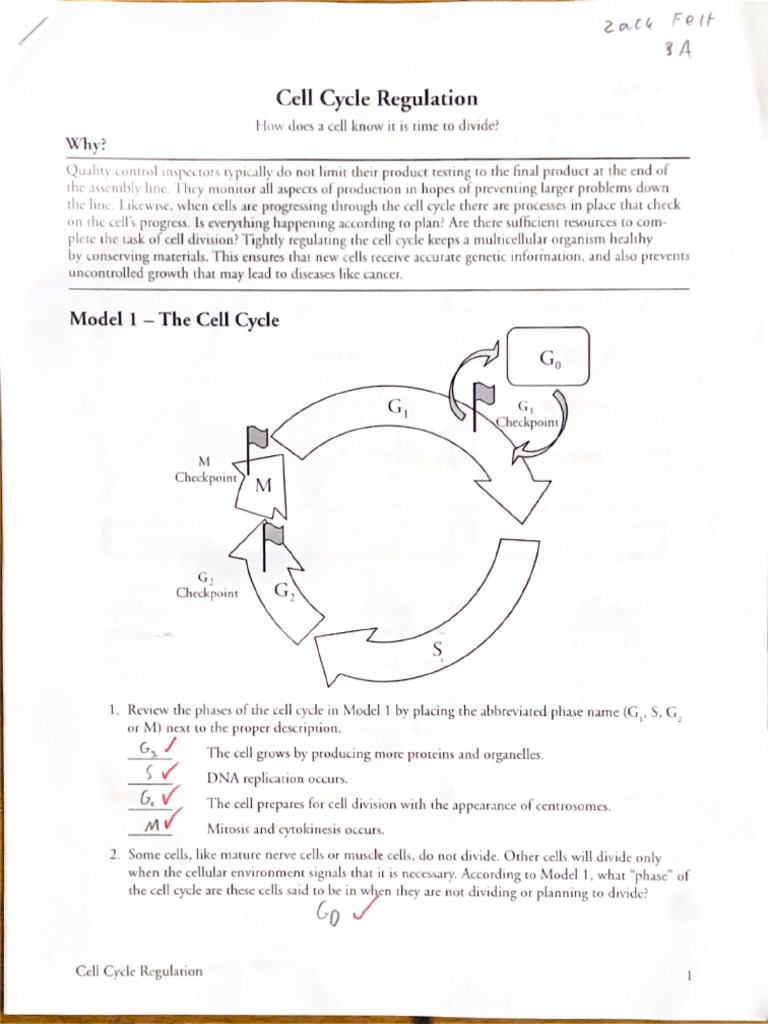
Understanding the Building Blocks of Matter: Atomic Structure
At the heart of every element lies its atomic structure, a concept that has fascinated scientists and researchers for centuries. The study of atomic structure has led to numerous breakthroughs in various fields, including chemistry, physics, and materials science. In this article, we will delve into the world of atomic structure, exploring its fundamental principles and key components.
The History of Atomic Structure
The concept of atomic structure dates back to ancient Greece, where philosophers such as Democritus proposed the idea of indivisible particles called atoms. However, it wasn’t until the 19th century that the modern understanding of atomic structure began to take shape. Scientists like John Dalton, J.J. Thomson, and Ernest Rutherford made significant contributions to our understanding of the atom, laying the foundation for the development of modern atomic theory.
The Components of Atomic Structure
An atom consists of three primary components: protons, neutrons, and electrons. Each of these components plays a crucial role in determining the properties of an element.
- Protons: Positively charged particles that reside in the nucleus, which is the central part of the atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms.
- Neutrons: Particles that have no charge and reside in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes of the same element.
- Electrons: Negatively charged particles that orbit the nucleus. The number of electrons in an atom is equal to the number of protons, and electrons are arranged in energy levels or shells around the nucleus.
Electron Configuration and the Periodic Table
Electron configuration refers to the arrangement of electrons in an atom. The periodic table is a tabular arrangement of elements, organized by their atomic number (number of protons), electron configuration, and recurring chemical properties. The periodic table is a powerful tool for understanding the relationships between elements and predicting their properties.
Atomic Structure and Chemical Bonding
The arrangement of electrons in an atom determines its chemical properties, including its ability to form bonds with other atoms. Chemical bonds are formed when electrons are shared or transferred between atoms, resulting in the formation of molecules. Understanding atomic structure is essential for understanding chemical bonding and the properties of molecules.
Atomic Structure and Nuclear Reactions
Atomic structure also plays a crucial role in nuclear reactions, which involve changes to the nucleus of an atom. Nuclear reactions can result in the formation of new elements, and understanding atomic structure is essential for predicting the outcomes of these reactions.
⚠️ Note: Understanding atomic structure is essential for understanding many phenomena in chemistry and physics, from chemical bonding to nuclear reactions.
In conclusion, atomic structure is a fundamental concept that underlies many areas of science and engineering. By understanding the components of atomic structure and how they interact, scientists and researchers can gain insights into the properties of elements and the behavior of molecules. From the periodic table to nuclear reactions, atomic structure plays a vital role in shaping our understanding of the world around us.
What is the main component of an atom?
+The main components of an atom are protons, neutrons, and electrons.
What determines the element of an atom?
+The number of protons in an atom determines the element of an atom.
What is the periodic table?
+The periodic table is a tabular arrangement of elements, organized by their atomic number, electron configuration, and recurring chemical properties.
Related Terms:
- How atoms differ answer key
- Subatomic Particles worksheet answer key
- Section 4.3 How atoms Differ