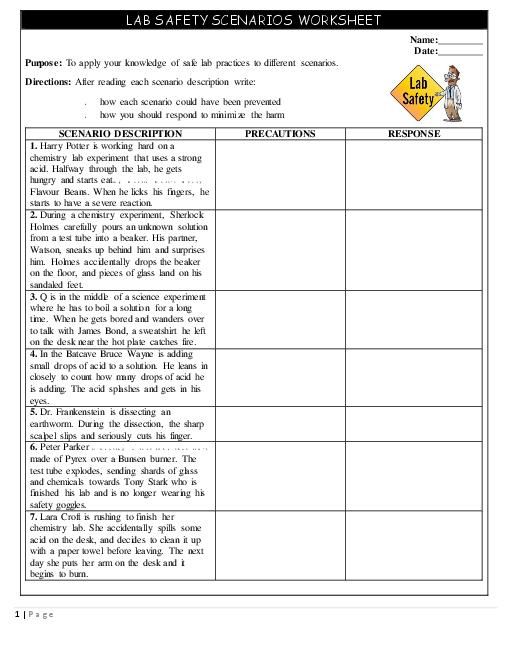
The Periodic Table Review Worksheet
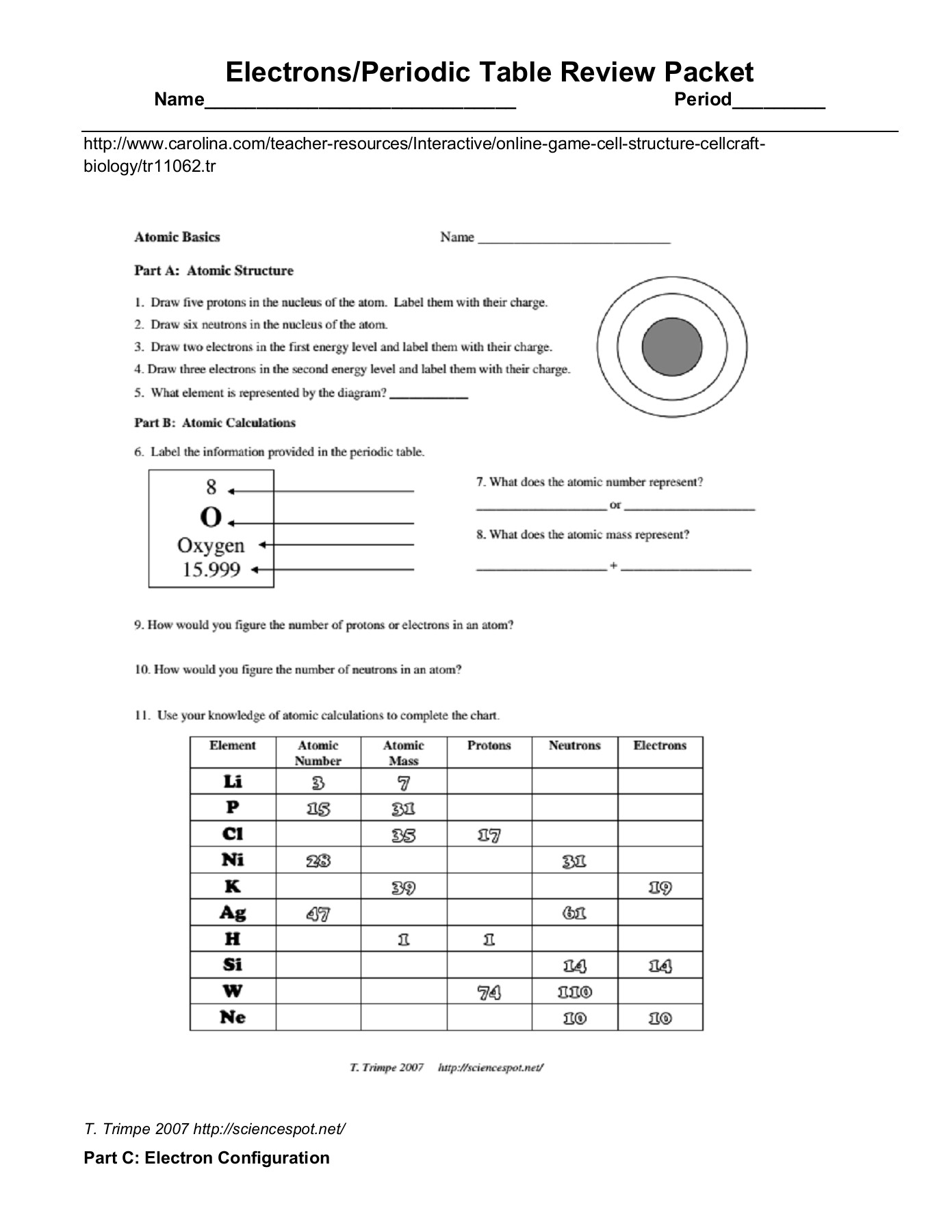
Understanding the Periodic Table: A Comprehensive Review
The periodic table is a fundamental tool in chemistry, used to organize and display the known elements. It is a powerful instrument for predicting the properties and behavior of elements, as well as understanding the relationships between them. In this review, we will delve into the structure and components of the periodic table, and explore its significance in the field of chemistry.
The Structure of the Periodic Table
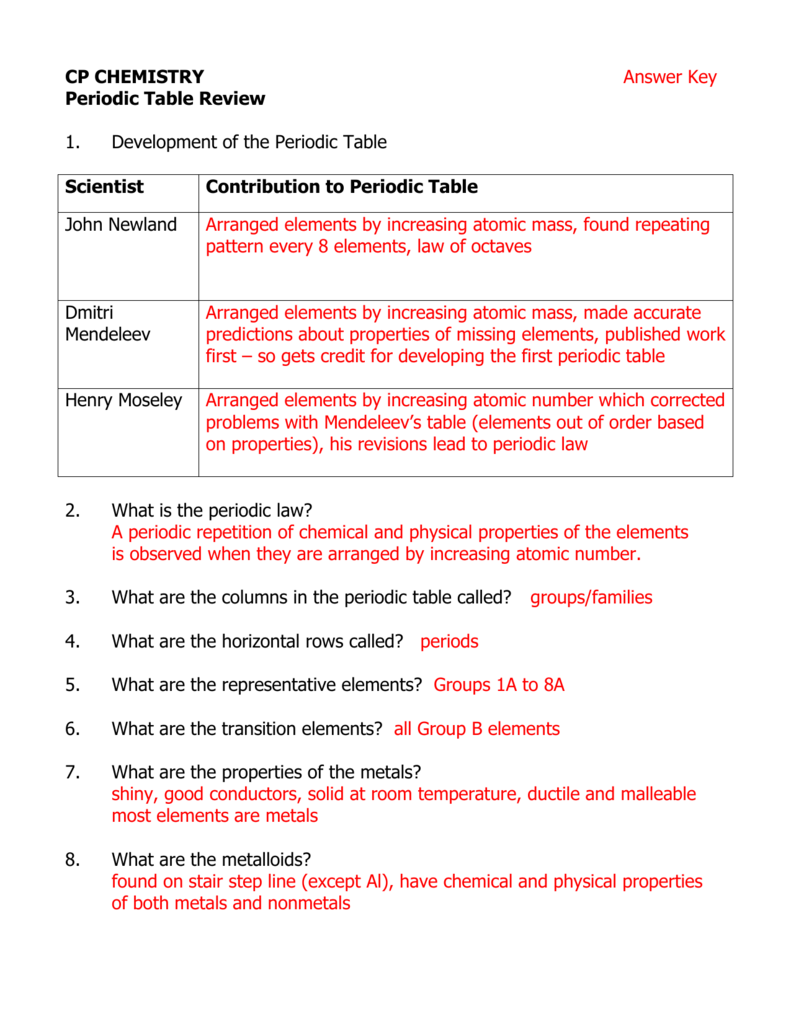
The periodic table is arranged in a logical and systematic way, with elements listed in order of increasing atomic number (number of protons in the nucleus). The table is divided into rows called periods and columns called groups or families. Elements in the same group exhibit similar chemical properties, while elements in the same period exhibit trends in their physical properties.
| Period | Group | Block |
|---|---|---|
| Rows of the periodic table | Columns of the periodic table | Regions of the periodic table |
The Components of the Periodic Table
The periodic table consists of several key components, including:
- Elements: The building blocks of matter, listed in order of increasing atomic number.
- Symbols: Abbreviations used to represent each element, usually consisting of one or two letters.
- Atomic Number: The number of protons in an element’s nucleus, which determines its position in the periodic table.
- Atomic Mass: The total number of protons and neutrons in an element’s nucleus.
- Groups: Columns of the periodic table, which exhibit similar chemical properties.
- Periods: Rows of the periodic table, which exhibit trends in physical properties.
The Blocks of the Periodic Table
The periodic table can be divided into several blocks, each corresponding to a specific region of the table. These blocks are:
- s-Block: Elements in the first two groups of the periodic table, which exhibit similar chemical properties.
- p-Block: Elements in the last six groups of the periodic table, which exhibit similar chemical properties.
- d-Block: Elements in the middle section of the periodic table, which exhibit similar chemical properties.
- f-Block: Elements in the bottom two rows of the periodic table, which exhibit similar chemical properties.
The Families of the Periodic Table
The periodic table can be divided into several families, each consisting of elements with similar chemical properties. These families include:
- Alkali Metals: Elements in Group 1 of the periodic table, which are highly reactive and exhibit similar chemical properties.
- Alkaline Earth Metals: Elements in Group 2 of the periodic table, which are less reactive than alkali metals but still exhibit similar chemical properties.
- Halogens: Elements in Group 17 of the periodic table, which are highly reactive and exhibit similar chemical properties.
- Noble Gases: Elements in Group 18 of the periodic table, which are unreactive and exhibit similar chemical properties.
The Significance of the Periodic Table
The periodic table is a powerful tool in chemistry, used to predict the properties and behavior of elements. It allows chemists to:
- Predict chemical properties: By identifying an element’s position in the periodic table, chemists can predict its chemical properties and behavior.
- Identify trends: The periodic table exhibits trends in physical properties, such as atomic radius and electronegativity.
- Understand relationships: The periodic table shows the relationships between elements, including their similarities and differences.
💡 Note: The periodic table is a dynamic tool, with new elements being added and existing elements being revised. It is essential to stay up-to-date with the latest developments in the field of chemistry.
📝 Note: The periodic table is a complex and intricate tool, and this review provides only a brief overview of its structure and components. For a more comprehensive understanding, it is recommended to consult a chemistry textbook or online resource.
In summary, the periodic table is a fundamental tool in chemistry, used to organize and display the known elements. Its structure and components allow chemists to predict the properties and behavior of elements, identify trends, and understand relationships between elements.
What is the periodic table?
+The periodic table is a tabular display of the known elements, organized by their atomic number, electron configuration, and recurring chemical properties.
What are the components of the periodic table?
+The periodic table consists of elements, symbols, atomic numbers, atomic masses, groups, periods, and blocks.
What is the significance of the periodic table?
+The periodic table is a powerful tool in chemistry, used to predict the properties and behavior of elements, identify trends, and understand relationships between elements.
Related Terms:
- Periodic table Review Worksheet PDF
- Periodic table review quizlet
- Periodic table families Worksheet PDF