5 Stoichiometry Mole to Mole Worksheet Answers
Understanding Stoichiometry: A Guide to Mole-to-Mole Calculations
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationship between the reactants and products in a chemical reaction. It involves calculating the amounts of substances required or produced in a reaction, which is crucial in various fields such as chemistry, physics, and engineering. In this article, we will focus on mole-to-mole calculations, which are a critical aspect of stoichiometry.
What is Stoichiometry?
Stoichiometry is the calculation of the quantities of reactants and products in a chemical reaction. It is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Stoichiometry involves the use of chemical equations to calculate the amounts of substances required or produced in a reaction.
What is a Mole?
A mole (mol) is a unit of measurement in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules. It is a fundamental concept in stoichiometry, as it allows us to calculate the amounts of substances required or produced in a reaction.
Mole-to-Mole Calculations
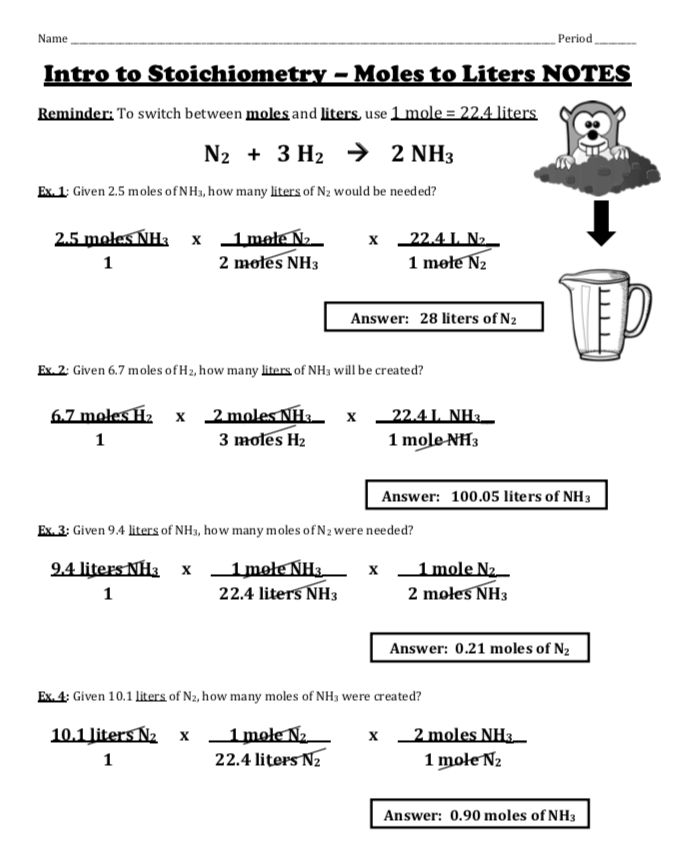
Mole-to-mole calculations involve converting between the number of moles of reactants and products in a chemical reaction. This is done using the mole ratio, which is the ratio of the number of moles of one substance to the number of moles of another substance.
Example 1: Mole-to-Mole Calculation
Consider the following chemical equation:
2H2 + O2 → 2H2O
What is the mole ratio of H2 to H2O?
To solve this problem, we need to determine the number of moles of H2 and H2O in the equation. The equation shows that 2 moles of H2 react with 1 mole of O2 to produce 2 moles of H2O.
Therefore, the mole ratio of H2 to H2O is 2:2 or 1:1.
Example 2: Mole-to-Mole Calculation
Consider the following chemical equation:
Ca + 2HCl → CaCl2 + H2
What is the mole ratio of Ca to HCl?
To solve this problem, we need to determine the number of moles of Ca and HCl in the equation. The equation shows that 1 mole of Ca reacts with 2 moles of HCl to produce 1 mole of CaCl2 and 1 mole of H2.
Therefore, the mole ratio of Ca to HCl is 1:2.
Solved Problems
Here are some solved problems to help you understand mole-to-mole calculations:
- Consider the following chemical equation:
2Al + Fe2O3 → 2Fe + Al2O3
What is the mole ratio of Al to Fe?
Answer: 2:2 or 1:1
- Consider the following chemical equation:
Na + Cl2 → NaCl
What is the mole ratio of Na to Cl2?
Answer: 1:1
- Consider the following chemical equation:
CaCO3 → CaO + CO2
What is the mole ratio of CaCO3 to CO2?
Answer: 1:1
- Consider the following chemical equation:
2K + 2H2O → 2KOH + H2
What is the mole ratio of K to H2O?
Answer: 1:1
- Consider the following chemical equation:
NH3 + HCl → NH4Cl
What is the mole ratio of NH3 to HCl?
Answer: 1:1
Notes
- Mole-to-mole calculations are used to determine the amounts of substances required or produced in a chemical reaction.
- The mole ratio is used to convert between the number of moles of reactants and products in a chemical reaction.
- Mole-to-mole calculations are based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Additional Resources
- Khan Academy: Stoichiometry
- Crash Course: Stoichiometry
- Chemistry LibreTexts: Stoichiometry
What is stoichiometry?
+Stoichiometry is the calculation of the quantities of reactants and products in a chemical reaction.
What is a mole?
+A mole is a unit of measurement in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules.
What is the mole ratio?
+The mole ratio is the ratio of the number of moles of one substance to the number of moles of another substance.
Why are mole-to-mole calculations important?
+Mole-to-mole calculations are used to determine the amounts of substances required or produced in a chemical reaction, which is crucial in various fields such as chemistry, physics, and engineering.
How are mole-to-mole calculations based on the law of conservation of mass?
+Mole-to-mole calculations are based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.