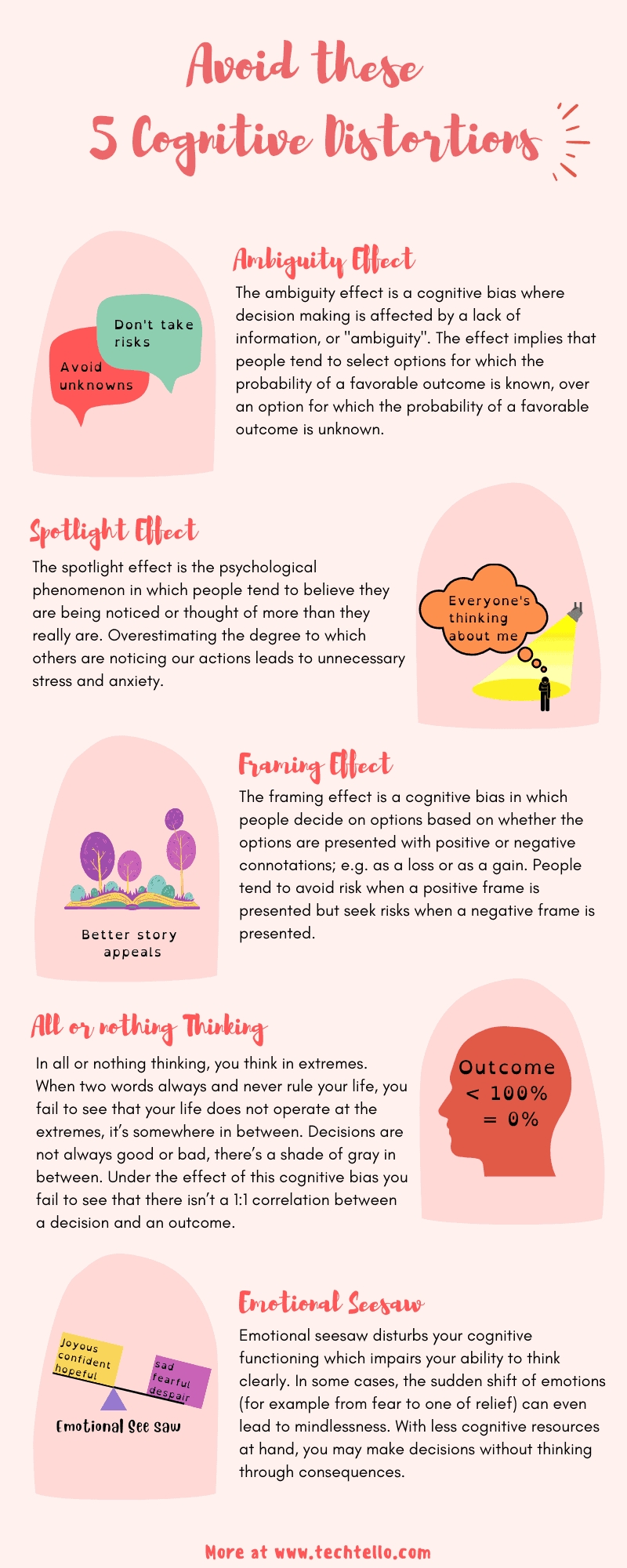
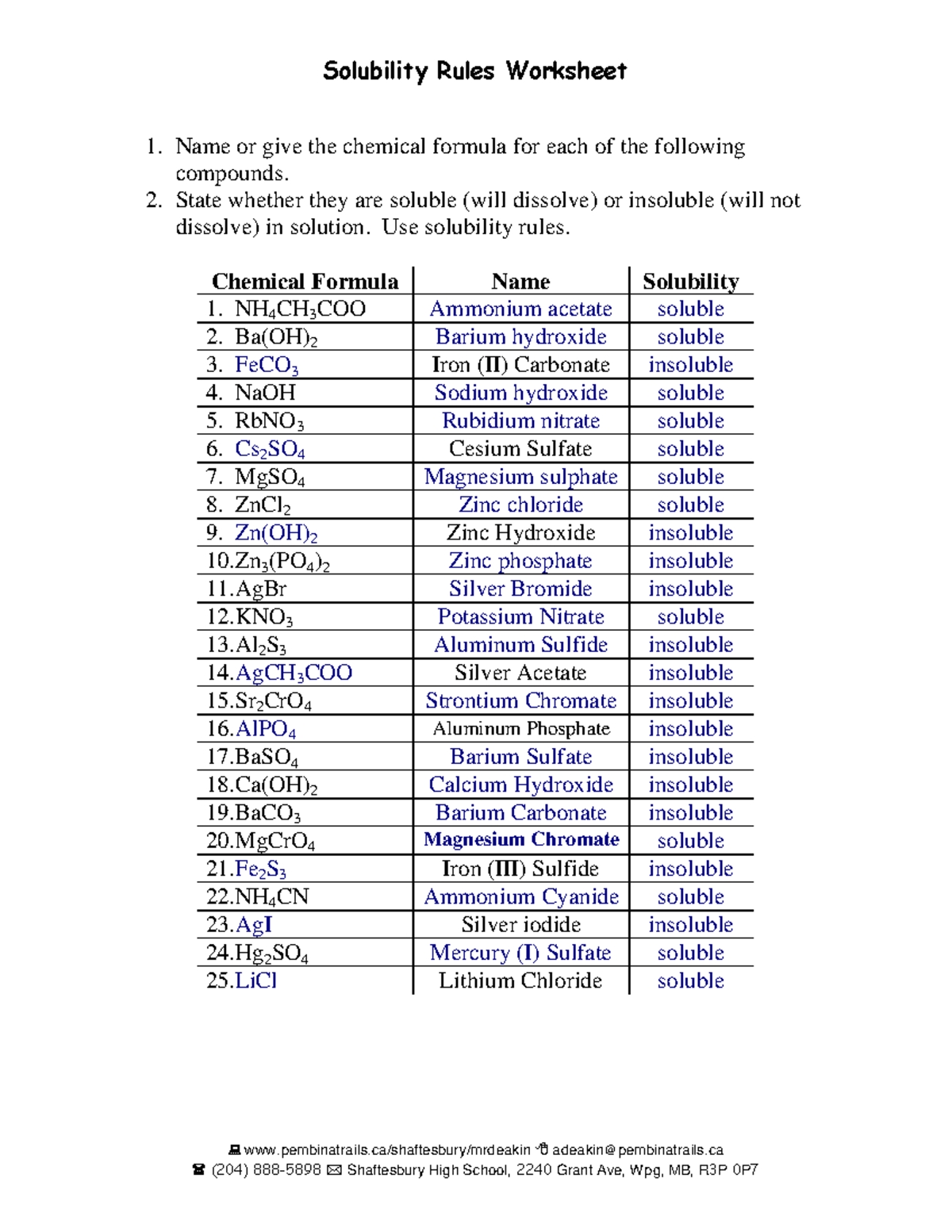
5 Ways to Master Solubility with Answer Key

Understanding Solubility
Solubility is a fundamental concept in chemistry that refers to the ability of a substance to dissolve in a given solvent. It is a crucial aspect of various chemical reactions, pharmaceutical applications, and environmental processes. Mastering solubility can help you better understand the behavior of different substances in various solvents. In this article, we will explore five ways to master solubility and provide you with a comprehensive answer key to help you solidify your understanding.
1. Learn the Solubility Rules
One of the most effective ways to master solubility is to learn the solubility rules. These rules provide a general guideline for predicting the solubility of different compounds in water and other solvents. Here are some key solubility rules to keep in mind:
- Most sodium, potassium, and ammonium salts are soluble.
- Most nitrates and acetates are soluble.
- Most chlorides are soluble, except for AgCl, PbCl2, and Hg2Cl2.
- Most sulfates are soluble, except for BaSO4, SrSO4, and PbSO4.
- Most carbonates are insoluble, except for those of alkali metals and ammonium.
📝 Note: These rules are not absolute and may have exceptions. However, they provide a good starting point for predicting solubility.
2. Understand the Factors Affecting Solubility
Several factors can influence the solubility of a substance in a given solvent. These factors include:
- Temperature: Generally, solubility increases with temperature.
- Pressure: Solubility is generally independent of pressure, except for gases.
- Concentration: Solubility can be affected by the concentration of the solvent.
- Surface Area: Increasing the surface area of the solute can increase solubility.
📊 Note: These factors can interact with each other in complex ways, so it's essential to consider them collectively when predicting solubility.
3. Practice Solubility Calculations
To master solubility, you need to practice calculating solubility values. Here’s a step-by-step guide to calculating solubility:
- Write the balanced equation: Write the balanced chemical equation for the dissolution reaction.
- Identify the solute and solvent: Identify the solute and solvent in the reaction.
- Calculate the molar concentration: Calculate the molar concentration of the solute using the formula: molarity = moles of solute / liters of solvent.
- Use the solubility product constant (Ksp): Use the Ksp value to calculate the solubility of the solute.
| Solute | Solubility Product Constant (Ksp) |
|---|---|
| AgCl | 1.8 x 10^-10 |
| PbCl2 | 1.7 x 10^-5 |
| BaSO4 | 1.1 x 10^-10 |
4. Use Solubility Tables and Graphs
Solubility tables and graphs can provide a quick and easy way to determine the solubility of different substances. Here are some common types of solubility tables and graphs:
- Solubility tables: Provide a list of solubility values for different substances.
- Solubility curves: Plot the solubility of a substance against temperature or concentration.
- Phase diagrams: Show the relationship between solubility and temperature or pressure.
5. Apply Solubility Concepts to Real-World Problems
To truly master solubility, you need to apply the concepts to real-world problems. Here are some examples:
- Pharmaceutical applications: Solubility is crucial in pharmaceutical applications, such as determining the solubility of drugs in water or other solvents.
- Environmental processes: Solubility plays a key role in environmental processes, such as determining the solubility of pollutants in water or soil.
- Chemical reactions: Solubility is essential in chemical reactions, such as determining the solubility of reactants and products.
And that’s it! By following these five steps and practicing regularly, you can master solubility and become a pro in no time.
In the next section, we’ll provide an answer key to help you solidify your understanding of solubility.
Answer Key
Here are the answers to some common solubility problems:
- What is the solubility of AgCl in water at 25°C? Answer: 1.8 x 10^-5 mol/L
- What is the solubility of PbCl2 in water at 25°C? Answer: 1.7 x 10^-5 mol/L
- What is the solubility of BaSO4 in water at 25°C? Answer: 1.1 x 10^-10 mol/L
We hope this article has helped you master solubility and provided you with a comprehensive understanding of this fundamental concept in chemistry.
What is solubility?
+Solubility is the ability of a substance to dissolve in a given solvent.
What are the solubility rules?
+The solubility rules provide a general guideline for predicting the solubility of different compounds in water and other solvents.
How does temperature affect solubility?
+Generally, solubility increases with temperature.
Related Terms:
- Solubility Worksheet answer key pdf
- solutions and solubility (worksheet answers)
- Solubility POGIL
- Solubility Curves Worksheet
- Solubility curve questions and answers
- Types of solutions Worksheet answers