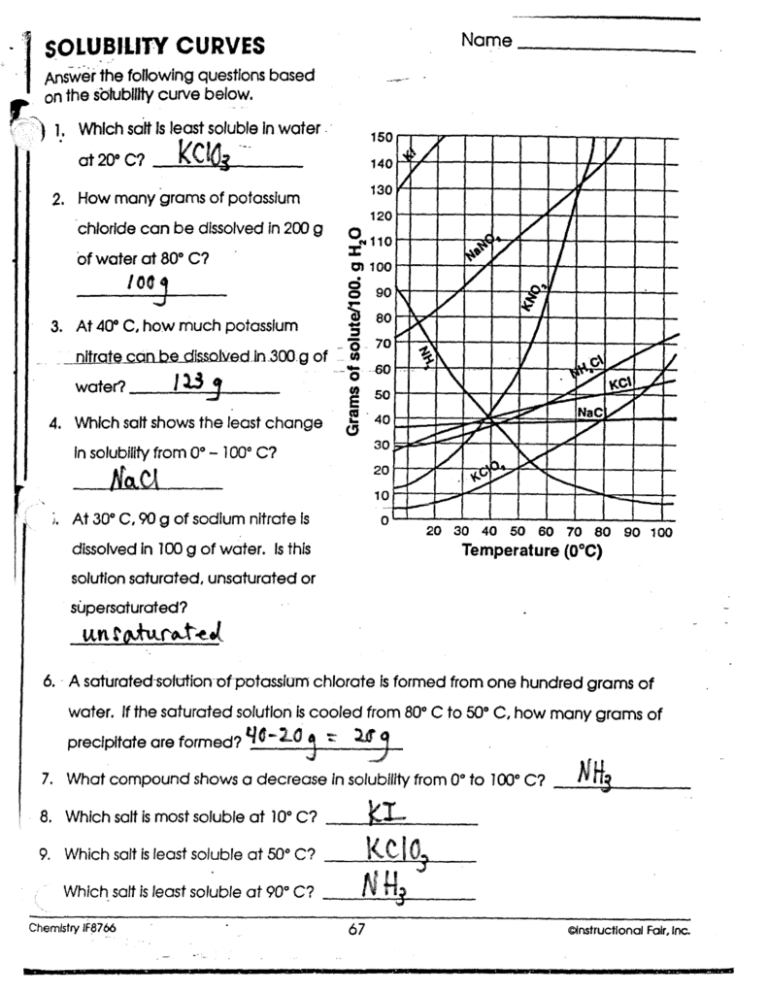
Solubility Curve Worksheet Answers
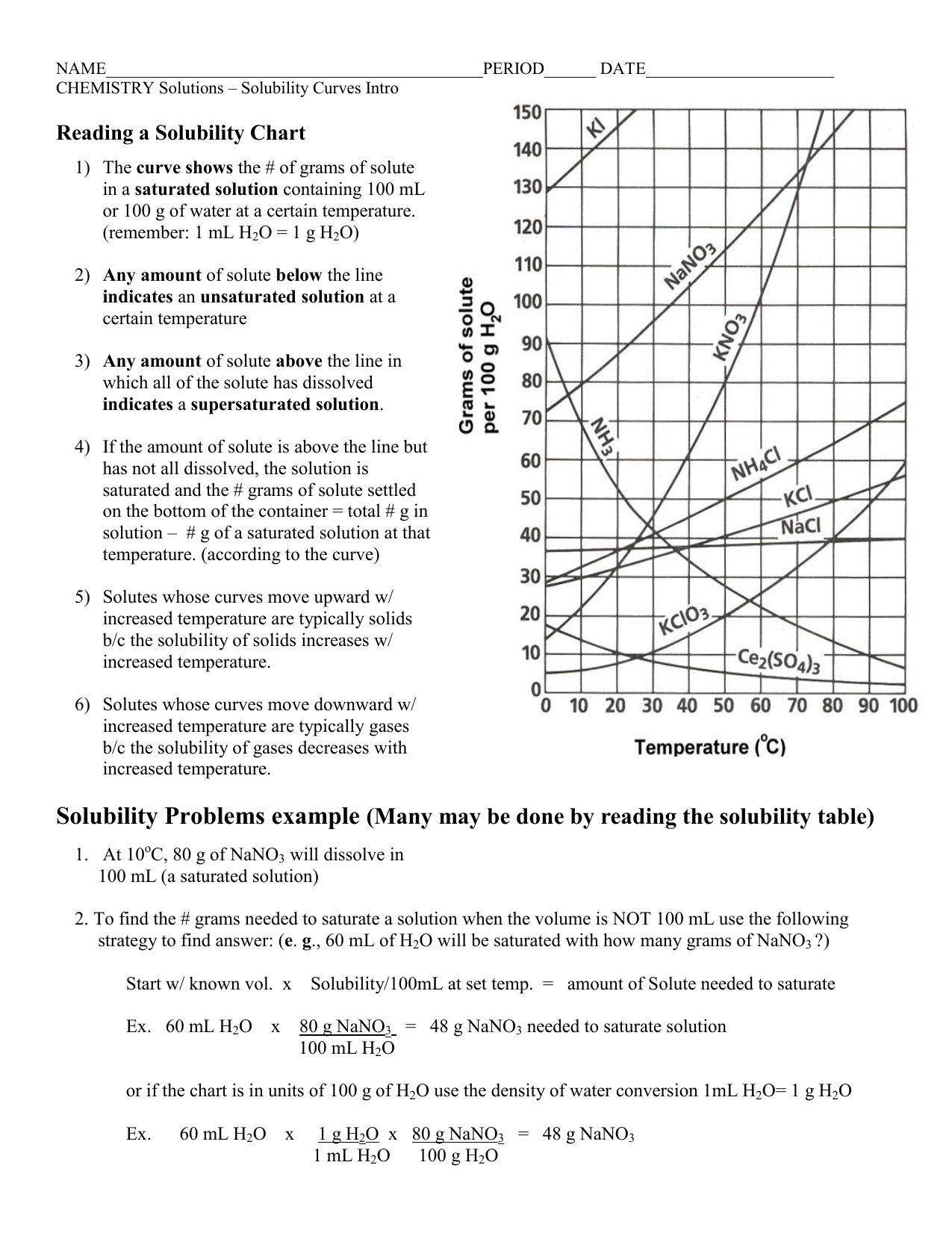
Solubility Curve Worksheet Answers
Solubility curves are graphical representations of the solubility of a substance in a solvent at various temperatures. Understanding and interpreting these curves is crucial in chemistry, particularly in predicting the behavior of substances in different conditions. Below, we will explore answers to common problems related to solubility curves, enhancing comprehension and application of these concepts.
Understanding Solubility Curves
A solubility curve is a graph that shows how the solubility of a substance in a given solvent changes with temperature. These curves can be used to determine the maximum amount of a substance that can dissolve in a solvent at a given temperature.
How to Read a Solubility Curve
- Identify the Axes: The x-axis represents temperature, and the y-axis represents the amount of substance that can dissolve in a solvent.
- Determine Solubility at a Given Temperature: To find the solubility of a substance at a specific temperature, locate the temperature on the x-axis and move upwards to the curve. The point where you intersect the curve corresponds to the solubility of the substance at that temperature on the y-axis.
- Identify the Type of Solubility Curve: Solubility curves can be classified based on their shapes and trends. A curve that goes upward indicates that the solubility increases with temperature. A downward curve indicates decreasing solubility with temperature, and a flat curve suggests that the solubility is constant over the temperature range shown.
Solving Problems with Solubility Curves
Example 1: Determining Solubility
[📝] Note: This is a fictional example for illustrative purposes.
Given a solubility curve for substance X, determine the maximum amount of X that can dissolve in 100g of water at 25°C.
- Solution: Locate 25°C on the x-axis and find the corresponding point on the curve. If the y-axis value at this point is 40g, then the maximum amount of X that can dissolve in 100g of water at 25°C is 40g.
Example 2: Understanding Temperature Effects
[❄️] Note: Understanding temperature effects is crucial for handling and storing substances.
What happens to the solubility of a substance as the temperature increases if its solubility curve slopes upward?
- Solution: If the solubility curve slopes upward, it means that the solubility of the substance increases with an increase in temperature.
Common Types of Solubility Curves
| Type of Curve | Description | Example |
|---|---|---|
| Increasing Curve | Solubility increases with temperature. | Sugar in water |
| Decreasing Curve | Solubility decreases with temperature. | Gas in a liquid under high pressure |
| Flat Curve | Solubility remains constant over the temperature range. | Some organic compounds in certain solvents |
Notes on Solubility Curves
[📊] Note: Always interpret solubility curves in the context of the specific substance and solvent mentioned.
- Metastable Equilibrium: Sometimes, a substance might not dissolve completely even if the temperature suggests higher solubility. This is due to metastable equilibrium, where the system is temporarily in a state of lower solubility.
- Solubility at Specific Points: Always read the temperature and solubility values directly from the curve or use the scale of the axes to estimate these values accurately.
Conclusion
Solubility curves are a powerful tool in chemistry for predicting the behavior of substances in various conditions. By understanding how to read and interpret these curves, chemists can make informed decisions about the handling, storage, and application of substances. Each curve provides unique insights into how temperature affects solubility, guiding experiments and industrial processes.
What does a solubility curve represent?
+A solubility curve represents the solubility of a substance in a solvent at various temperatures, showing how the amount of substance that can dissolve changes with temperature.
How do you determine the solubility of a substance at a given temperature from a solubility curve?
+To determine the solubility, locate the temperature on the x-axis, move upwards to the curve, and read the solubility value from the y-axis.
What does an upward sloping solubility curve indicate?
+An upward sloping curve indicates that the solubility of the substance increases with an increase in temperature.