Solubility Curve Worksheet 2 Answer Key
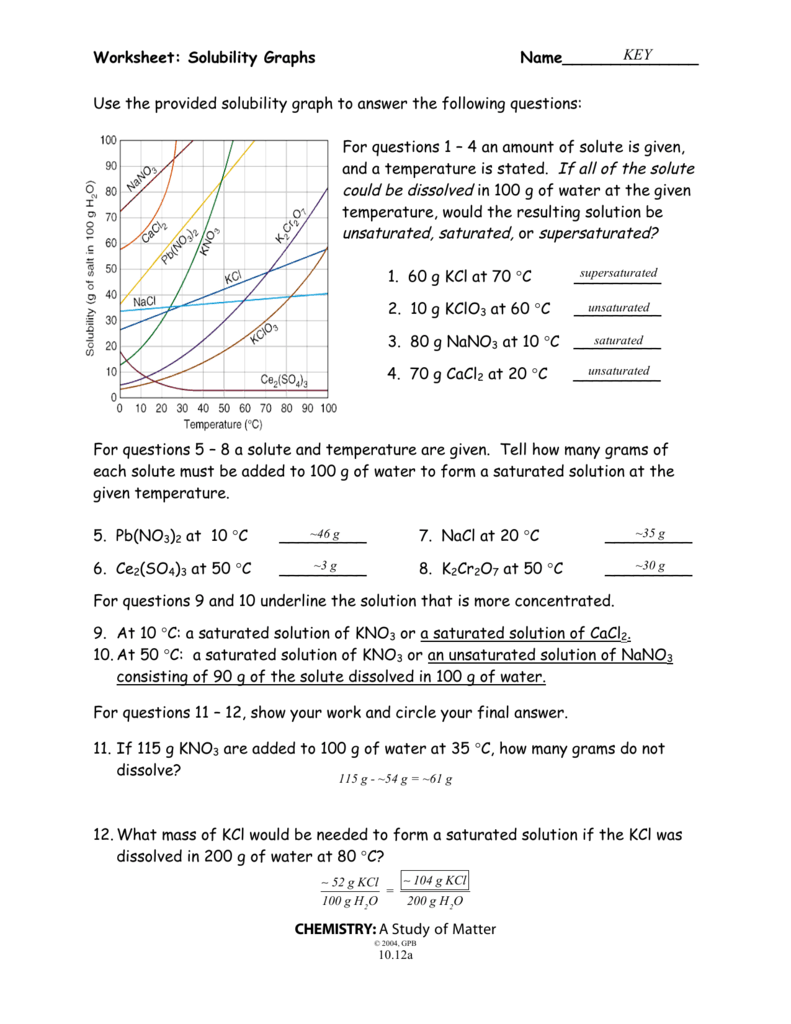
Solubility Curve Worksheet 2 Answer Key
Welcome to the Solubility Curve Worksheet 2 Answer Key! This worksheet is designed to help you understand and work with solubility curves, which are essential in chemistry. Below, you’ll find answers to questions that will guide you through understanding solubility curves, how to read them, and how to interpret the data they provide.
What is a Solubility Curve?
A solubility curve is a graphical representation showing how the solubility of a substance changes with temperature. It’s a powerful tool for chemists and researchers to understand the behavior of substances in various conditions.
Interpreting Solubility Curves
When interpreting solubility curves, it’s essential to understand what the different parts of the graph represent:
- Solubility: The amount of substance that can dissolve in a given amount of solvent at a particular temperature.
- Temperature: The independent variable, typically plotted on the x-axis.
- Saturation Point: The point at which the solution becomes saturated with the substance and cannot dissolve any more.
Types of Solubility Curves
Solubility curves can be classified into several types based on their shape and behavior:
- Normal Solubility Curve: The curve slopes upward to the right, indicating that solubility increases with temperature.
- Inverse Solubility Curve: The curve slopes downward to the right, indicating that solubility decreases with temperature.
- Retrosgrade Solubility Curve: The curve initially slopes upward but then changes direction and slopes downward, indicating a decrease in solubility after a certain temperature.
Example Solubility Curve Questions and Answers
Question 1
A solubility curve for a substance shows an upward slope. What does this indicate?
Answer: This indicates that the solubility of the substance increases with temperature.
Question 2
At what point on the solubility curve is the solution saturated?
Answer: The solution becomes saturated at the saturation point on the curve, beyond which no more substance can dissolve.
Question 3
What does a retrosgrade solubility curve signify?
Answer: It signifies that after a certain temperature, the solubility of the substance decreases.
Worksheet Questions

| Question Number | Question | Answer |
|---|---|---|
| 1 | Describe a scenario where knowing the solubility curve of a substance would be crucial. | Knowing the solubility curve is crucial in industrial processes where substances need to be dissolved in solvents under varying temperature conditions to achieve optimal production. |
| 2 | What is the significance of the point where the solubility curve meets the temperature axis? | This point indicates the temperature below which the substance does not dissolve in the solvent. |
| 3 | Why do some substances have an inverse solubility curve? | Some substances have an inverse solubility curve because their solubility decreases with an increase in temperature, often due to the specific interactions between the substance and the solvent molecules. |
[🔍] Note: These questions are meant to be used as a guide and may vary based on the specific worksheet provided.
Understanding Solubility Curve Implications
Solubility curves have implications in various fields, including chemistry, environmental science, and pharmaceuticals. Understanding these curves can help in:
- Optimizing Industrial Processes: By knowing the optimal temperature for dissolving substances, industries can save energy and resources.
- Predicting Environmental Fate: Solubility curves can help predict how substances will behave in different environmental conditions, aiding in the assessment of their potential impact.
- Designing Medications: In pharmaceuticals, solubility curves are crucial for designing drugs that are efficiently absorbed by the body.
Conclusion
Understanding solubility curves is essential for anyone working with substances in various conditions. These curves provide valuable information on how the solubility of a substance changes with temperature, aiding in the optimization of processes, prediction of environmental fate, and design of medications. By grasping the concept of solubility curves and how to interpret them, one can make more informed decisions in a range of scientific and industrial contexts.
What is the purpose of a solubility curve?
+The purpose of a solubility curve is to show how the solubility of a substance changes with temperature.
How do you read a solubility curve?
+To read a solubility curve, you look at the x-axis for the temperature and the y-axis for the solubility. The curve’s shape indicates how solubility changes with temperature.
What does an upward sloping solubility curve indicate?
+An upward sloping solubility curve indicates that the solubility of the substance increases with temperature.
Related Terms:
- Solubility Worksheet answer Key
- Solubility Curves Practice 2
- Solubility curve questions and answers
- Solubility curve PDF
- Solubility temperature Graphs Worksheet answers