5 Ways to Master Solubility Curve Worksheet
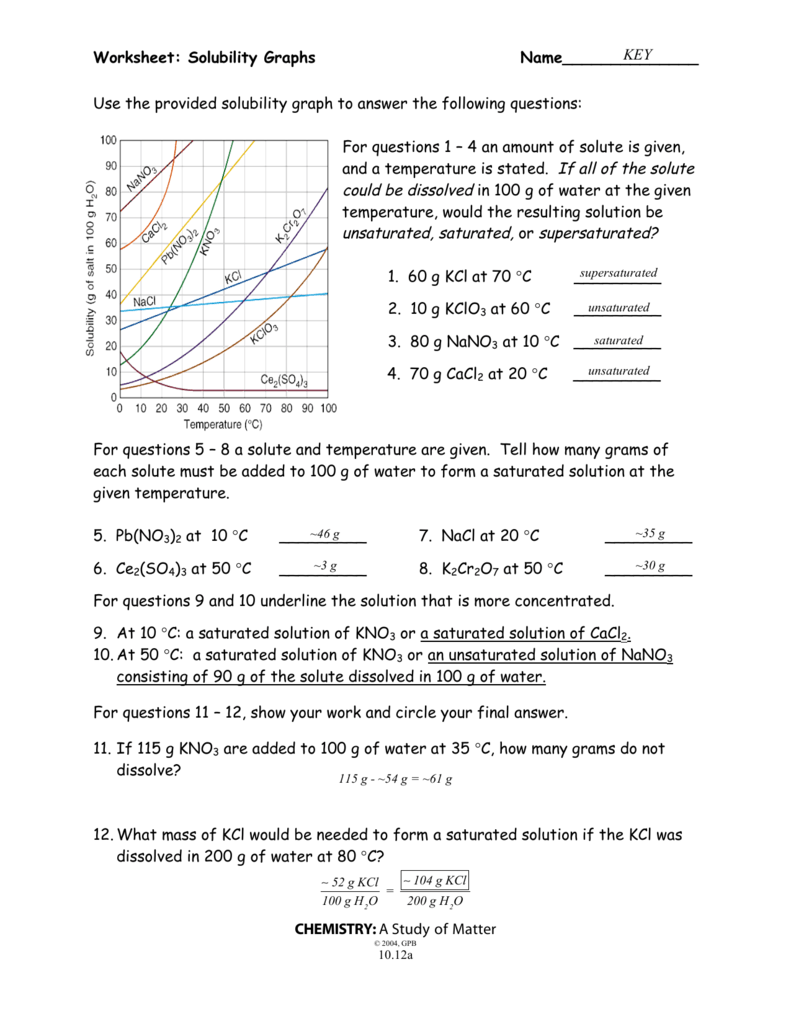
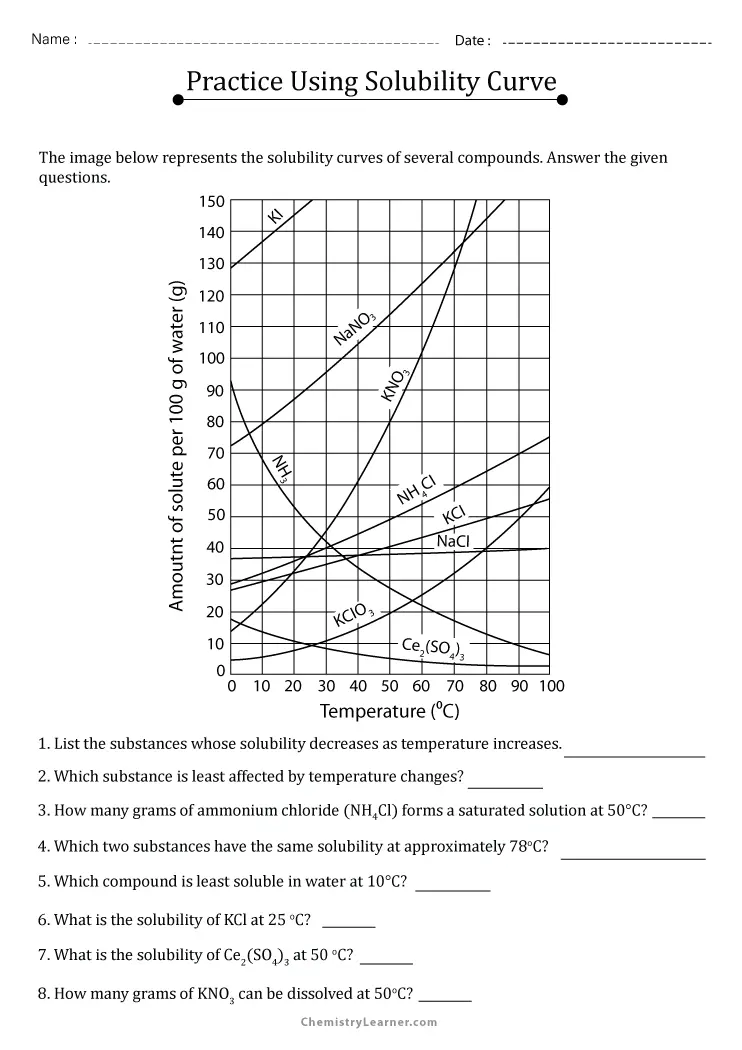
Understanding Solubility Curve Worksheet
Solubility curve worksheet is a crucial tool used in chemistry to understand the relationship between the solubility of a substance and temperature. It is a graphical representation of the amount of substance that can dissolve in a given amount of solvent at different temperatures. Mastering the solubility curve worksheet is essential for students and professionals in the field of chemistry, as it helps them to predict the behavior of substances in various solutions.
Why is Solubility Curve Worksheet Important?
The solubility curve worksheet is important because it helps to:
- Predict Solubility: By analyzing the solubility curve, you can predict the maximum amount of substance that can dissolve in a given amount of solvent at a specific temperature.
- Understand Phase Changes: The solubility curve helps to understand the phase changes that occur in a substance as the temperature changes.
- Determine Solvent Choice: By analyzing the solubility curve, you can determine the best solvent to use for a particular substance.
5 Ways to Master Solubility Curve Worksheet
Here are five ways to master the solubility curve worksheet:
1. Understand the Basics
To master the solubility curve worksheet, you need to understand the basics of solubility and the factors that affect it. Solubility is the ability of a substance to dissolve in a solvent. The solubility of a substance is affected by temperature, pressure, and the nature of the solvent.
- Temperature: An increase in temperature generally increases the solubility of a substance.
- Pressure: An increase in pressure generally decreases the solubility of a substance.
- Nature of Solvent: The solubility of a substance is affected by the nature of the solvent. For example, polar substances are more soluble in polar solvents.
2. Read and Interpret the Graph
To master the solubility curve worksheet, you need to be able to read and interpret the graph. The graph is a plot of the solubility of a substance against temperature.
- Identify the Axes: The x-axis represents the temperature, and the y-axis represents the solubility.
- Identify the Curve: The curve represents the relationship between the solubility and temperature.
- Identify the Points: The points on the curve represent the solubility at a specific temperature.
3. Analyze the Shape of the Curve
The shape of the curve can provide valuable information about the solubility of a substance.
- Increasing Solubility: If the curve is increasing, it means that the solubility of the substance increases with temperature.
- Decreasing Solubility: If the curve is decreasing, it means that the solubility of the substance decreases with temperature.
- Maximum Solubility: If the curve reaches a maximum point, it means that the solubility of the substance reaches a maximum value at a specific temperature.
4. Determine the Solubility at a Specific Temperature
To determine the solubility at a specific temperature, you need to read the value from the graph.
- Locate the Temperature: Locate the temperature on the x-axis.
- Read the Solubility: Read the solubility value from the y-axis.
5. Practice with Examples
To master the solubility curve worksheet, you need to practice with examples.
- Worked Examples: Work through examples to practice reading and interpreting the graph.
- Past Papers: Practice with past papers to get a feel for the types of questions that may be asked.
💡 Note: The more you practice, the more comfortable you will become with reading and interpreting the solubility curve worksheet.
Common Mistakes to Avoid
Here are some common mistakes to avoid when working with the solubility curve worksheet:
- Confusing the Axes: Make sure to identify the x-axis and y-axis correctly.
- Misreading the Curve: Make sure to read the curve correctly and identify the shape of the curve.
- Rounding Errors: Make sure to round your answers correctly to avoid errors.
| Temperature (°C) | Solubility (g/100mL) |
|---|---|
| 20 | 10 |
| 30 | 20 |
| 40 | 30 |
By following these tips and practicing with examples, you can master the solubility curve worksheet and become more confident in your ability to predict the solubility of substances.
By mastering the solubility curve worksheet, you will be able to predict the behavior of substances in various solutions, determine the best solvent to use for a particular substance, and understand the phase changes that occur in a substance as the temperature changes.
What is the solubility curve worksheet?
+The solubility curve worksheet is a graphical representation of the amount of substance that can dissolve in a given amount of solvent at different temperatures.
What are the factors that affect solubility?
+The factors that affect solubility are temperature, pressure, and the nature of the solvent.
How do I determine the solubility at a specific temperature?
+To determine the solubility at a specific temperature, you need to read the value from the graph by locating the temperature on the x-axis and reading the solubility value from the y-axis.