6 Tips for Understanding Solids Liquids and Gases
Understanding the Basics of Solids, Liquids, and Gases
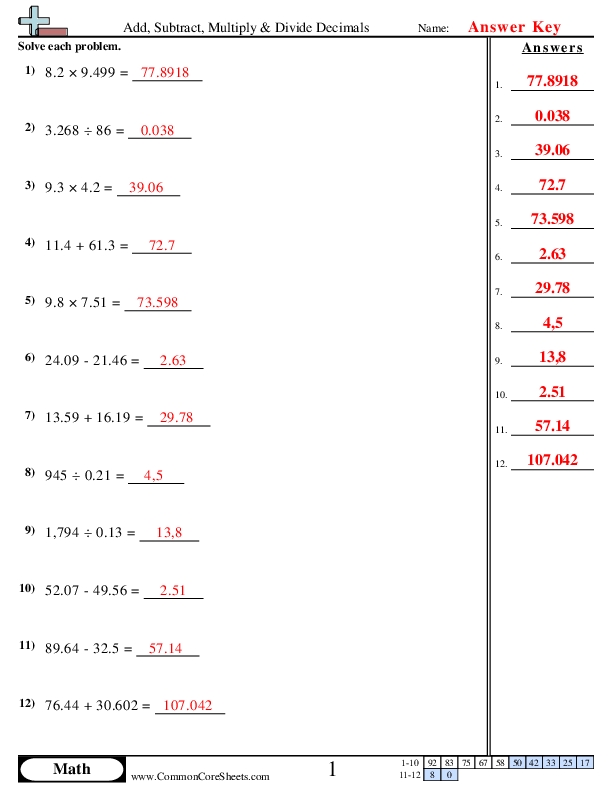
The three main states of matter - solids, liquids, and gases - are a fundamental concept in physics and chemistry. Understanding the properties and behaviors of each state is crucial for grasping various scientific principles and phenomena. In this article, we will delve into the characteristics of solids, liquids, and gases, and provide six tips for understanding these states of matter.
Characteristics of Solids
Solids have a fixed shape and volume. The particles in a solid are closely packed and have a regular structure, which gives the solid its rigidity. The particles in a solid vibrate in place but do not change their position. Examples of solids include rocks, metals, and ice.
Characteristics of Liquids
Liquids have a fixed volume but take the shape of their container. The particles in a liquid are close together but are free to move past each other. This property allows liquids to flow and change shape. Examples of liquids include water, oil, and juice.
Characteristics of Gases
Gases have neither a fixed shape nor a fixed volume. The particles in a gas are widely spaced and are free to move in any direction. This property allows gases to expand and fill their container. Examples of gases include air, helium, and steam.
Tip 1: Visualize the Particle Arrangement
To understand the properties of solids, liquids, and gases, it’s essential to visualize the arrangement of particles in each state. Imagine the particles as balls that are either closely packed (solids), close but able to move (liquids), or widely spaced (gases). This visualization will help you understand how the particles interact with each other and their surroundings.
Tip 2: Understand the Concept of Phase Changes
Phase changes occur when a substance changes from one state of matter to another. For example, when ice (solid) melts, it becomes water (liquid). When water is heated, it turns into steam (gas). Understanding phase changes will help you appreciate the dynamic nature of the states of matter.
Tip 3: Recognize the Importance of Temperature and Pressure
Temperature and pressure play a crucial role in determining the state of matter. Increasing the temperature of a substance can cause it to change from a solid to a liquid to a gas. Similarly, increasing the pressure on a substance can cause it to change from a gas to a liquid to a solid.
Tip 4: Use Real-Life Examples to Illustrate the States of Matter
Using real-life examples can help make the concept of states of matter more relatable and easier to understand. For instance, think of a ice cube (solid) melting into water (liquid) as it’s left at room temperature. Or, imagine a balloon filled with air (gas) that can be compressed to a smaller size by increasing the pressure.
Tip 5: Learn About the Properties of Different States of Matter
Each state of matter has unique properties that distinguish it from the others. For example, solids have a fixed shape and volume, while liquids take the shape of their container. Gases, on the other hand, have neither a fixed shape nor a fixed volume. Understanding these properties will help you appreciate the characteristics of each state of matter.
Tip 6: Practice, Practice, Practice!
Practice is key to understanding the states of matter. Try to identify the state of matter of various substances in your daily life. Ask yourself questions like “Is this substance a solid, liquid, or gas?” or “What would happen if I change the temperature or pressure of this substance?” The more you practice, the more comfortable you’ll become with the concept of states of matter.
💡 Note: Understanding the states of matter is a fundamental concept in physics and chemistry. It's essential to build a strong foundation in this concept to appreciate more advanced scientific principles.
As we conclude our discussion on the states of matter, remember that understanding these concepts is essential for grasping various scientific principles and phenomena. By following these six tips, you’ll be well on your way to becoming proficient in identifying and understanding the properties of solids, liquids, and gases.
What is the main difference between a solid and a liquid?
+The main difference between a solid and a liquid is the arrangement of particles. In a solid, the particles are closely packed and have a fixed position, whereas in a liquid, the particles are close together but are free to move past each other.
What is an example of a phase change?
+An example of a phase change is when ice (solid) melts into water (liquid). This occurs when the temperature of the ice is increased, causing the particles to gain energy and move more freely.
What is the importance of temperature and pressure in determining the state of matter?
+Temperature and pressure play a crucial role in determining the state of matter. Increasing the temperature of a substance can cause it to change from a solid to a liquid to a gas, while increasing the pressure on a substance can cause it to change from a gas to a liquid to a solid.