Shapes of Molecules Worksheet Answers
Molecules come in a variety of shapes, and understanding these shapes is crucial in chemistry. Here’s a comprehensive guide to help you navigate the world of molecular shapes, along with answers to common questions and a FAQ section at the end.
Understanding Molecular Shapes
The shape of a molecule is determined by the arrangement of its atoms in space. This arrangement is crucial for understanding the chemical and physical properties of a substance. The shape of a molecule can affect its polarity, reactivity, and even its biological activity.
Types of Molecular Shapes
Molecules can be classified into several types based on their shapes. Here are some of the most common types:
- Linear: In a linear molecule, the atoms are arranged in a straight line. An example of a linear molecule is carbon dioxide (CO2).
- Bent: In a bent molecule, the atoms are arranged in a V-shape. An example of a bent molecule is water (H2O).
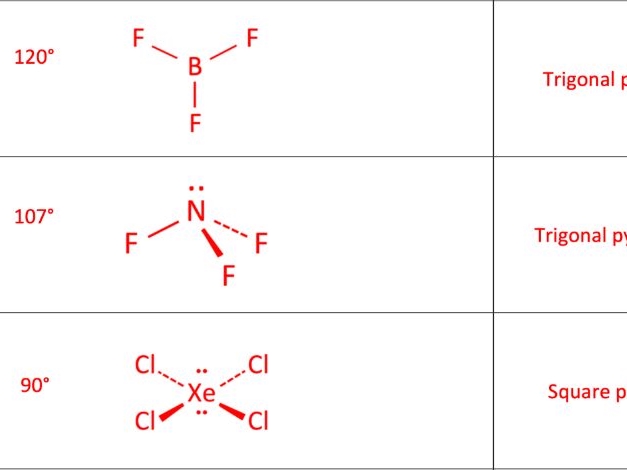
- Trigonal Planar: In a trigonal planar molecule, the atoms are arranged in a triangular shape. An example of a trigonal planar molecule is boron trifluoride (BF3).
- Tetrahedral: In a tetrahedral molecule, the atoms are arranged in a four-sided pyramid shape. An example of a tetrahedral molecule is methane (CH4).
- Octahedral: In an octahedral molecule, the atoms are arranged in an eight-sided shape. An example of an octahedral molecule is sulfur hexafluoride (SF6).
Determining Molecular Shapes
To determine the shape of a molecule, you need to follow these steps:
- Draw the Lewis Structure: Draw the Lewis structure of the molecule, which shows the arrangement of electrons in the molecule.
- Count the Number of Electron Groups: Count the number of electron groups around the central atom. Electron groups can be either bonded atoms or lone pairs.
- Apply VSEPR Theory: Apply the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron groups around a central atom will arrange themselves to minimize repulsions between them.
- Determine the Molecular Shape: Based on the arrangement of electron groups, determine the molecular shape.
Worksheets and Answers
Here are some worksheets and answers to help you practice determining molecular shapes:
Worksheet 1: Linear Molecules
| Molecule | Lewis Structure | Electron Groups | Molecular Shape |
|---|---|---|---|
| CO2 | O=C=O | 2 | Linear |
| HCN | H-C≡N | 2 | Linear |
Answers:
- CO2: Linear
- HCN: Linear
Worksheet 2: Bent Molecules
| Molecule | Lewis Structure | Electron Groups | Molecular Shape |
|---|---|---|---|
| H2O | H-O-H | 4 | Bent |
| SO2 | O=S=O | 3 | Bent |
Answers:
- H2O: Bent
- SO2: Bent
Worksheet 3: Trigonal Planar Molecules
| Molecule | Lewis Structure | Electron Groups | Molecular Shape |
|---|---|---|---|
| BF3 | F-B-F | 3 | Trigonal Planar |
| CO3^2- | O=C=O | 3 | Trigonal Planar |
Answers:
- BF3: Trigonal Planar
- CO3^2-: Trigonal Planar
Notes
🔍 Note: When determining molecular shapes, it's essential to consider the arrangement of electron groups around the central atom. This will help you predict the shape of the molecule accurately.
🔍 Note: VSEPR theory is a useful tool for predicting molecular shapes. However, it's essential to remember that it's a simplification and may not always accurately predict the shape of complex molecules.
What is the difference between a linear and a bent molecule?
+A linear molecule has a straight line arrangement of atoms, while a bent molecule has a V-shape arrangement of atoms.
How do I determine the molecular shape of a molecule?
+To determine the molecular shape, draw the Lewis structure, count the number of electron groups, apply VSEPR theory, and determine the molecular shape based on the arrangement of electron groups.
What is VSEPR theory?
+VSEPR (Valence Shell Electron Pair Repulsion) theory states that electron groups around a central atom will arrange themselves to minimize repulsions between them.
In conclusion, understanding molecular shapes is crucial in chemistry, and following these steps can help you determine the shape of a molecule accurately. By practicing with worksheets and applying VSEPR theory, you can become proficient in predicting molecular shapes.