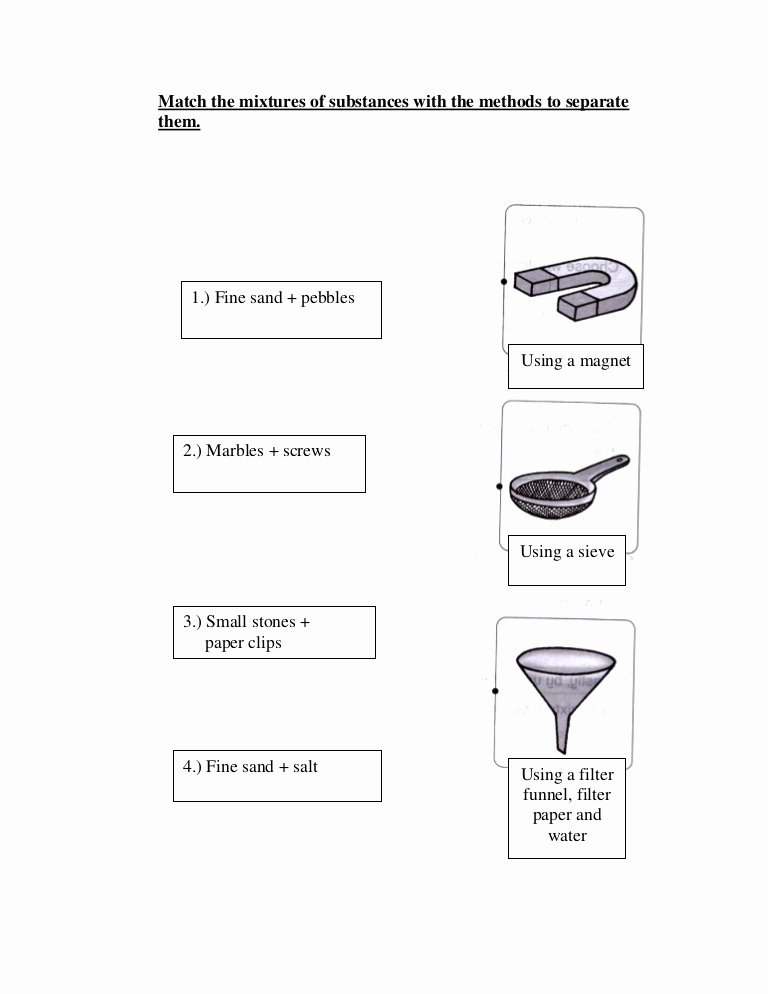
Separation Of Mixtures Worksheet
Separation of Mixtures: A Comprehensive Guide
Mixtures are an essential part of our daily lives, and separating them is a crucial process in various industries such as chemistry, biology, and environmental science. In this article, we will delve into the world of separation of mixtures, exploring the different methods, techniques, and applications.
What is a Mixture?
A mixture is a physical blend of two or more substances, where each substance retains its chemical properties. Mixtures can be homogeneous, where the composition is uniform throughout, or heterogeneous, where the composition varies from one point to another.
Why Separate Mixtures?
Separating mixtures is essential for various reasons:
- Purification: Separating mixtures allows us to obtain pure substances, which is crucial in many industrial processes.
- Analysis: Separation helps us to analyze the composition of a mixture, which is vital in fields like chemistry and biology.
- Recycling: Separating mixtures enables us to recycle materials, reducing waste and conserving natural resources.
Methods of Separation
There are several methods of separating mixtures, including:
- Filtration: Using a porous material to separate solid particles from a liquid or gas.
- Decantation: Carefully pouring a liquid from a container, leaving the solid particles behind.
- Centrifugation: Using a centrifuge to separate solid particles from a liquid or gas.
- Evaporation: Heating a mixture to evaporate the liquid, leaving the solid particles behind.
- Distillation: Heating a mixture to separate the components based on their boiling points.
- Chromatography: Using a stationary phase to separate the components of a mixture based on their interactions with the phase.
- Magnetism: Using a magnet to separate magnetic materials from non-magnetic materials.
Techniques of Separation
Some common techniques used in separation of mixtures include:
- Column Chromatography: Using a vertical column to separate the components of a mixture.
- Paper Chromatography: Using a paper strip to separate the components of a mixture.
- Thin Layer Chromatography: Using a thin layer of stationary phase to separate the components of a mixture.
- Gas Chromatography: Using a gas as the mobile phase to separate the components of a mixture.
Applications of Separation of Mixtures
Separation of mixtures has numerous applications in various fields, including:
- Chemical Industry: Separation of mixtures is used to purify chemicals, separate reaction products, and recycle materials.
- Biotechnology: Separation of mixtures is used to purify biological molecules, separate cells, and analyze biological samples.
- Environmental Science: Separation of mixtures is used to analyze and monitor environmental samples, separate pollutants, and recycle materials.
Separation of Mixtures Worksheet
Here’s a worksheet to help you practice separation of mixtures:
Question 1: A mixture of sand and water is separated using filtration. What is the purpose of the filter paper?
A) To separate the sand from the water B) To dissolve the sand in the water C) To heat the mixture D) To cool the mixture
Answer: A) To separate the sand from the water
Question 2: A mixture of two liquids with different boiling points is separated using distillation. What is the purpose of the distillation apparatus?
A) To mix the two liquids B) To heat the mixture C) To separate the two liquids based on their boiling points D) To cool the mixture
Answer: C) To separate the two liquids based on their boiling points
Question 3: A mixture of a solid and a liquid is separated using centrifugation. What is the purpose of the centrifuge?
A) To mix the solid and liquid B) To heat the mixture C) To separate the solid from the liquid D) To cool the mixture
Answer: C) To separate the solid from the liquid
📝 Note: The answers to the worksheet questions can be found at the end of this article.
Conclusion
Separation of mixtures is a vital process in various industries and fields, and understanding the different methods and techniques is essential for effective separation. By practicing the separation of mixtures worksheet, you can improve your knowledge and skills in this area.
What is the difference between a mixture and a solution?
+A mixture is a physical blend of two or more substances, where each substance retains its chemical properties. A solution, on the other hand, is a homogeneous mixture of two or more substances, where the particles are molecularly dispersed.
What is the purpose of chromatography in separation of mixtures?
+Chromatography is used to separate the components of a mixture based on their interactions with a stationary phase. It is commonly used to separate and analyze the components of a mixture.
What is the difference between distillation and evaporation?
+Distillation is a separation process that involves heating a mixture to separate the components based on their boiling points. Evaporation, on the other hand, is a separation process that involves heating a mixture to evaporate the liquid, leaving the solid particles behind.
Answers to Worksheet Questions:
- A) To separate the sand from the water
- C) To separate the two liquids based on their boiling points
- C) To separate the solid from the liquid
Related Terms:
- Separating Mixtures Worksheet answers
- Separating mixtures worksheet KS3
- Separating mixtures Worksheet GCSE
- Separating mixtures activity
- KS3 mixtures worksheet
- Separating mixtures liveworksheets