6 Ways to Master Reaction Types
Understanding Reaction Types: A Comprehensive Guide
When it comes to chemistry, understanding reaction types is crucial for any student or enthusiast. Reaction types are the building blocks of chemistry, and mastering them can help you solve complex problems and understand the underlying principles of chemical reactions. In this article, we will explore six ways to master reaction types, including understanding the basics, practicing with examples, and using online resources.
1. Understand the Basics
Before diving into the different reaction types, it’s essential to understand the basics of chemical reactions. A chemical reaction is a process in which one or more substances (reactants) are converted into new substances (products). There are several key concepts to understand, including:
- Reactants: The substances that are consumed in the reaction.
- Products: The substances that are formed in the reaction.
- Catalysts: Substances that speed up the reaction without being consumed.
- Activation energy: The energy required for the reaction to occur.
Understanding these basic concepts will provide a solid foundation for learning about reaction types.
2. Learn the Main Reaction Types
There are several main reaction types, including:
- Synthesis reactions: Two or more reactants combine to form a new product.
- Decomposition reactions: A single reactant breaks down into two or more products.
- Replacement reactions: One reactant is replaced by another reactant.
- Combustion reactions: A reactant combines with oxygen to produce heat and light.
- Neutralization reactions: An acid and a base react to form a salt and water.
Each reaction type has its own unique characteristics and examples.
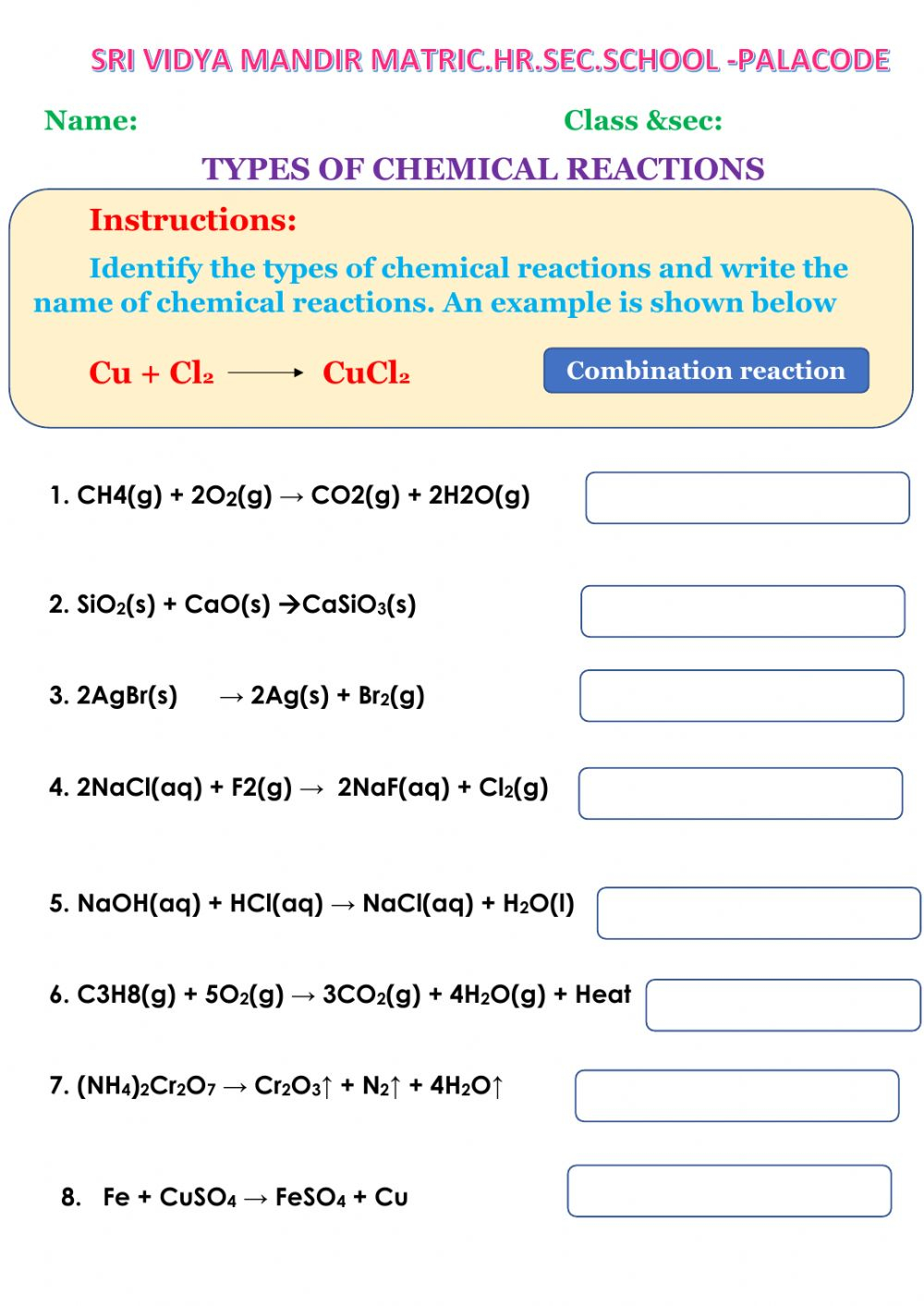
3. Practice with Examples
Practicing with examples is one of the best ways to master reaction types. Try to identify the reaction type for each example, and then balance the equation. Here are a few examples to get you started:
- Synthesis reaction: 2H2 + O2 → 2H2O
- Decomposition reaction: 2H2O → 2H2 + O2
- Replacement reaction: Zn + CuSO4 → ZnSO4 + Cu
Start with simple examples and gradually move on to more complex ones.
4. Use Online Resources
There are many online resources available to help you master reaction types. Some popular options include:
- Khan Academy: Offers video tutorials and practice exercises on reaction types.
- ChemGuide: Provides detailed explanations and examples of reaction types.
- ChemTube3D: Offers interactive 3D animations of chemical reactions.
These resources can supplement your learning and provide additional practice opportunities.
5. Watch Video Tutorials
Video tutorials can be an excellent way to learn about reaction types. Here are a few popular YouTube channels:
- Crash Course: Offers video tutorials on chemistry, including reaction types.
- 3Blue1Brown: Provides animated explanations of chemical reactions.
- AsapSCIENCE: Offers short, animated videos on chemistry topics, including reaction types.
Watching video tutorials can help you visualize the reactions and make them more memorable.
6. Take Online Quizzes
Taking online quizzes can help you assess your knowledge and identify areas for improvement. Here are a few popular options:
- Quizlet: Offers online quizzes and flashcards on reaction types.
- Chemistry LibreTexts: Provides online quizzes and practice exercises on reaction types.
- Khan Academy: Offers online quizzes and practice exercises on reaction types.
Taking online quizzes can help you reinforce your learning and build confidence.
💡 Note: Mastering reaction types takes time and practice. Be patient and persistent, and you will see improvement over time.
In conclusion, mastering reaction types requires a combination of understanding the basics, practicing with examples, and using online resources. By following these six tips, you can improve your knowledge and confidence in reaction types and become a chemistry master.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction involves two or more reactants combining to form a new product, while a decomposition reaction involves a single reactant breaking down into two or more products.
What is the role of a catalyst in a chemical reaction?
+A catalyst is a substance that speeds up a chemical reaction without being consumed in the process.
How can I practice identifying reaction types?
+Try identifying the reaction type for each example, and then balance the equation. You can also use online resources, such as Khan Academy or ChemGuide, to practice identifying reaction types.