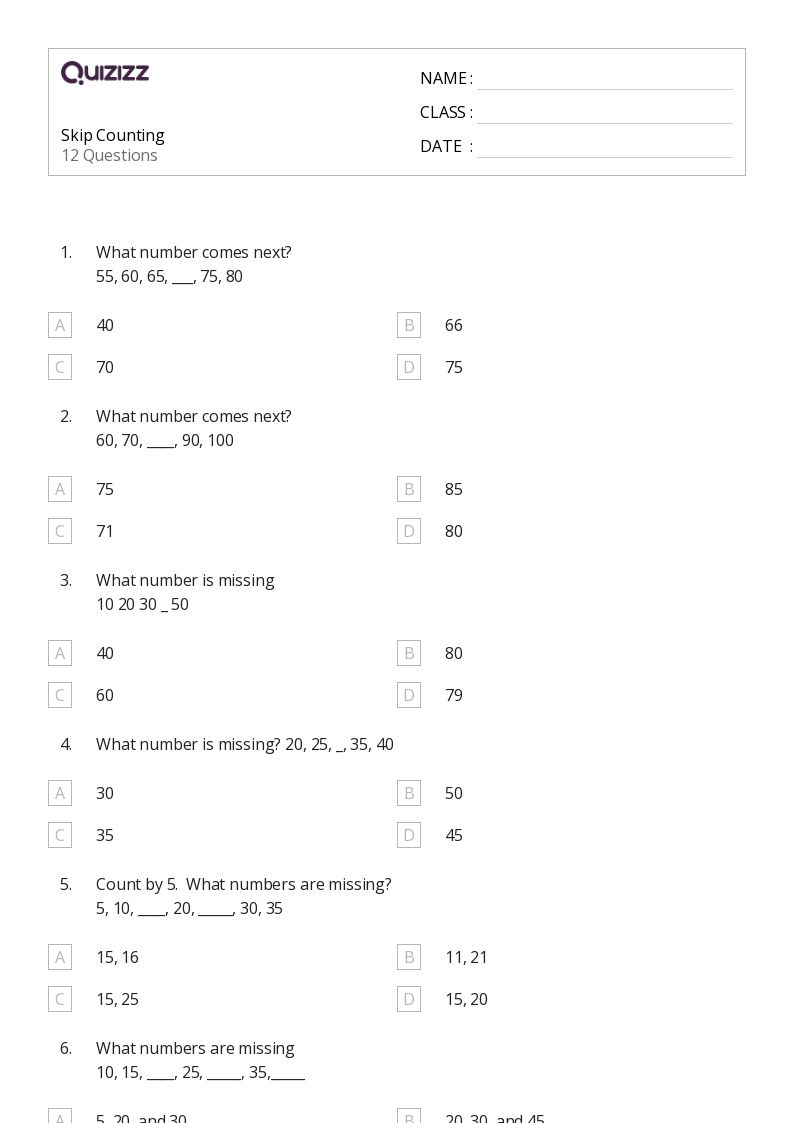
Chem 10 Review: Mastering Reaction Stoichiometry Made Easy
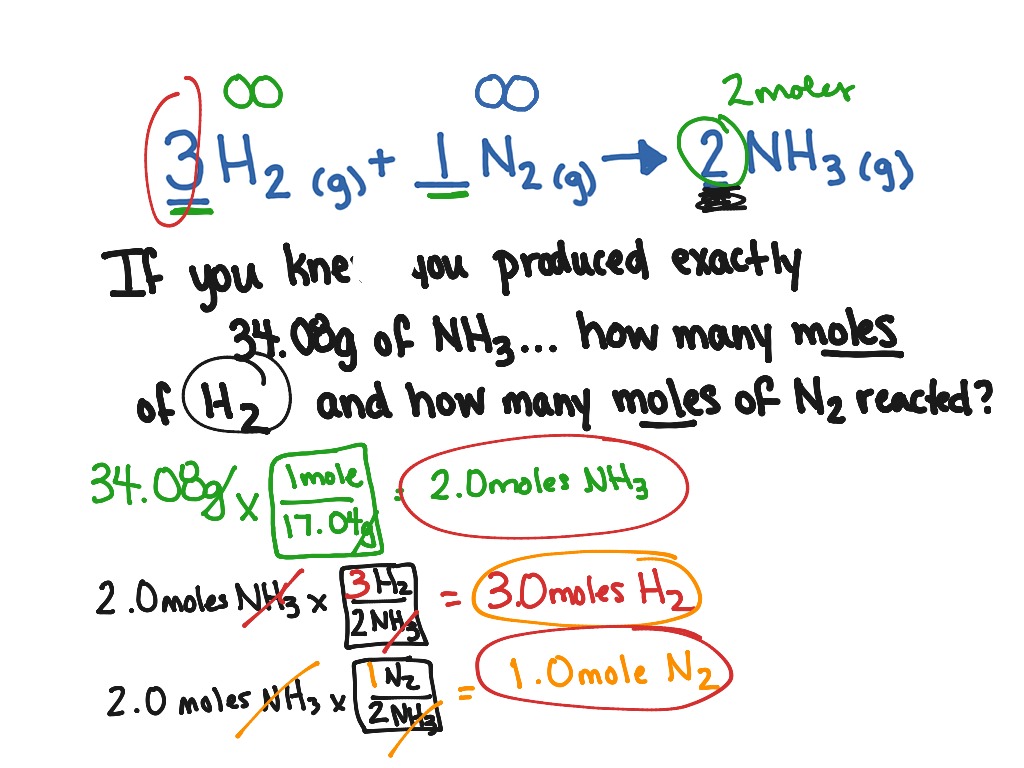
Understanding the Basics of Reaction Stoichiometry
Reaction stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It is a crucial aspect of chemistry, as it allows us to predict the amount of products that can be formed from a given amount of reactants, and vice versa. In this article, we will delve into the world of reaction stoichiometry and explore the key concepts and techniques that will help you master this subject.
The Mole Concept
The mole concept is the foundation of reaction stoichiometry. A mole is defined as the amount of a substance that contains as many particles (atoms or molecules) as there are atoms in 0.012 kilograms of carbon-12. This number is known as the Avogadro’s number, which is approximately 6.022 x 10^23 particles.
The mole concept is essential in reaction stoichiometry because it allows us to convert between the mass of a substance and the number of particles it contains. This is crucial in calculating the amount of reactants and products in a chemical reaction.
Balanced Chemical Equations
A balanced chemical equation is a mathematical representation of a chemical reaction that shows the ratio of reactants to products. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, so the number of atoms of each element must be the same on both the reactant and product sides of the equation.
Balanced chemical equations are essential in reaction stoichiometry because they provide a visual representation of the quantitative relationships between the reactants and products. By analyzing a balanced chemical equation, we can determine the mole ratio of reactants to products, which is crucial in calculating the amount of products that can be formed from a given amount of reactants.
Stoichiometric Calculations
Stoichiometric calculations involve using the mole ratio of reactants to products to calculate the amount of products that can be formed from a given amount of reactants. There are several types of stoichiometric calculations, including:
- Mass-mass calculations: These calculations involve using the mole ratio of reactants to products to calculate the mass of products that can be formed from a given mass of reactants.
- Mass-volume calculations: These calculations involve using the mole ratio of reactants to products to calculate the volume of products that can be formed from a given mass of reactants.
- Volume-volume calculations: These calculations involve using the mole ratio of reactants to products to calculate the volume of products that can be formed from a given volume of reactants.
Step-by-Step Guide to Stoichiometric Calculations
- Write the balanced chemical equation: Start by writing the balanced chemical equation for the reaction.
- Identify the given information: Identify the given information, such as the mass or volume of the reactants.
- Identify the unknown information: Identify the unknown information, such as the mass or volume of the products.
- Use the mole ratio to calculate the unknown information: Use the mole ratio of reactants to products to calculate the unknown information.
🔥 Note: When performing stoichiometric calculations, always use the mole ratio of reactants to products, not the ratio of masses or volumes.
Limits of Reactants
In some chemical reactions, one of the reactants may be present in excess, while another reactant is limiting. The limiting reactant is the reactant that determines the amount of products that can be formed.
To determine the limiting reactant, we need to calculate the mole ratio of reactants to products and compare it to the given information. The reactant that is present in the smallest amount relative to the mole ratio is the limiting reactant.
Percent Yield
Percent yield is the ratio of the actual yield of a product to the theoretical yield. The theoretical yield is the maximum amount of product that can be formed from a given amount of reactants, based on the mole ratio of reactants to products.
Percent yield is an important concept in reaction stoichiometry because it allows us to evaluate the efficiency of a chemical reaction. A high percent yield indicates that the reaction is efficient, while a low percent yield indicates that the reaction is not efficient.
Calculating Percent Yield
- Calculate the theoretical yield: Calculate the theoretical yield of the product based on the mole ratio of reactants to products.
- Calculate the actual yield: Calculate the actual yield of the product.
- Calculate the percent yield: Calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100.
📝 Note: When calculating percent yield, always use the actual yield and theoretical yield in the same units.
In summary, mastering reaction stoichiometry requires a deep understanding of the mole concept, balanced chemical equations, stoichiometric calculations, limits of reactants, and percent yield. By following the step-by-step guide to stoichiometric calculations and using the mole ratio of reactants to products, you can become proficient in calculating the amount of products that can be formed from a given amount of reactants.
What is the mole concept?
+The mole concept is the foundation of reaction stoichiometry. A mole is defined as the amount of a substance that contains as many particles (atoms or molecules) as there are atoms in 0.012 kilograms of carbon-12.
What is a balanced chemical equation?
+A balanced chemical equation is a mathematical representation of a chemical reaction that shows the ratio of reactants to products. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, so the number of atoms of each element must be the same on both the reactant and product sides of the equation.
What is the limiting reactant?
+The limiting reactant is the reactant that determines the amount of products that can be formed. To determine the limiting reactant, we need to calculate the mole ratio of reactants to products and compare it to the given information.