Protons Neutrons Electrons Worksheet Answer Key and Study Guide
Protons, Neutrons, Electrons: Understanding the Building Blocks of Atoms
Atoms are the fundamental units of matter, and they consist of three main subatomic particles: protons, neutrons, and electrons. Understanding the roles and relationships of these particles is crucial for mastering chemistry and physics. In this study guide, we will delve into the world of protons, neutrons, and electrons, and provide a comprehensive worksheet answer key to help you reinforce your knowledge.
What are Protons?
Protons are positively charged subatomic particles that reside in the nucleus of an atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. For example, hydrogen has one proton, helium has two protons, and oxygen has eight protons.
What are Neutrons?
Neutrons are subatomic particles that have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.
What are Electrons?
Electrons are negatively charged subatomic particles that orbit the nucleus of an atom. The number of electrons in a neutral atom is equal to the number of protons. Electrons are arranged in energy levels or shells around the nucleus, and each shell has a specific capacity for electrons.
Atomic Number and Mass Number
The atomic number of an element is the number of protons in an atom, which defines the element. The mass number is the total number of protons and neutrons in an atom. The mass number is used to calculate the atomic mass of an element.
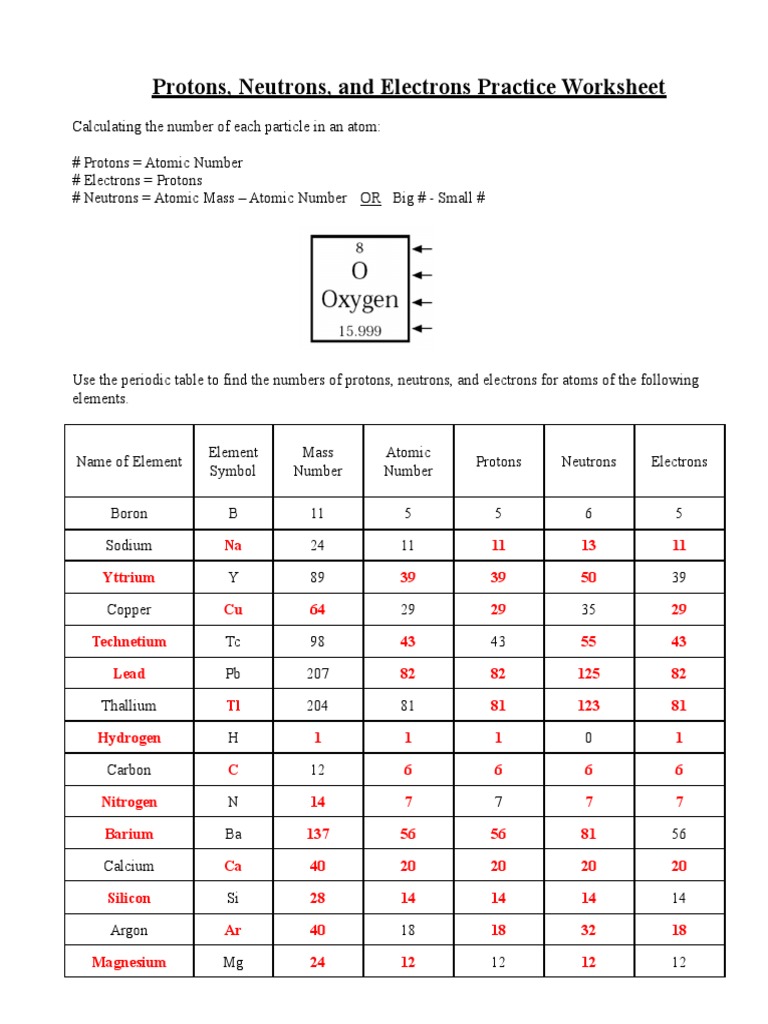
Worksheet Answer Key
What is the number of protons in the nucleus of a hydrogen atom? Answer: 1
What is the number of neutrons in the nucleus of a carbon-12 atom? Answer: 6
What is the number of electrons in a neutral helium atom? Answer: 2
What is the atomic number of oxygen? Answer: 8
What is the mass number of a carbon-14 atom? Answer: 14
What is the number of protons in the nucleus of a uranium-238 atom? Answer: 92
What is the number of electrons in a neutral lithium atom? Answer: 3
What is the atomic number of nitrogen? Answer: 7
What is the mass number of a hydrogen-2 atom? Answer: 2
What is the number of neutrons in the nucleus of a oxygen-16 atom? Answer: 8
Notes
📝 Note: The number of protons in an atom determines the element, while the number of neutrons can vary, leading to different isotopes.
📝 Note: The atomic number is the number of protons in an atom, while the mass number is the total number of protons and neutrons.
Study Tips
- Make sure to memorize the atomic numbers of common elements.
- Understand the relationship between protons, neutrons, and electrons in an atom.
- Practice calculating the mass number of an atom using the number of protons and neutrons.
- Review the concept of isotopes and how they differ from one another.
Conclusion
In conclusion, understanding the roles and relationships of protons, neutrons, and electrons is essential for mastering chemistry and physics. By using this study guide and worksheet answer key, you will be well-equipped to tackle more advanced topics in chemistry and physics. Remember to practice regularly and review the concepts to reinforce your knowledge.
What is the difference between atomic number and mass number?
+
The atomic number is the number of protons in an atom, while the mass number is the total number of protons and neutrons.
What is the number of electrons in a neutral atom?
+
The number of electrons in a neutral atom is equal to the number of protons.
What is an isotope?
+
An isotope is an atom of the same element that has the same number of protons but different numbers of neutrons.



