Printable Periodic Table Worksheet for Students
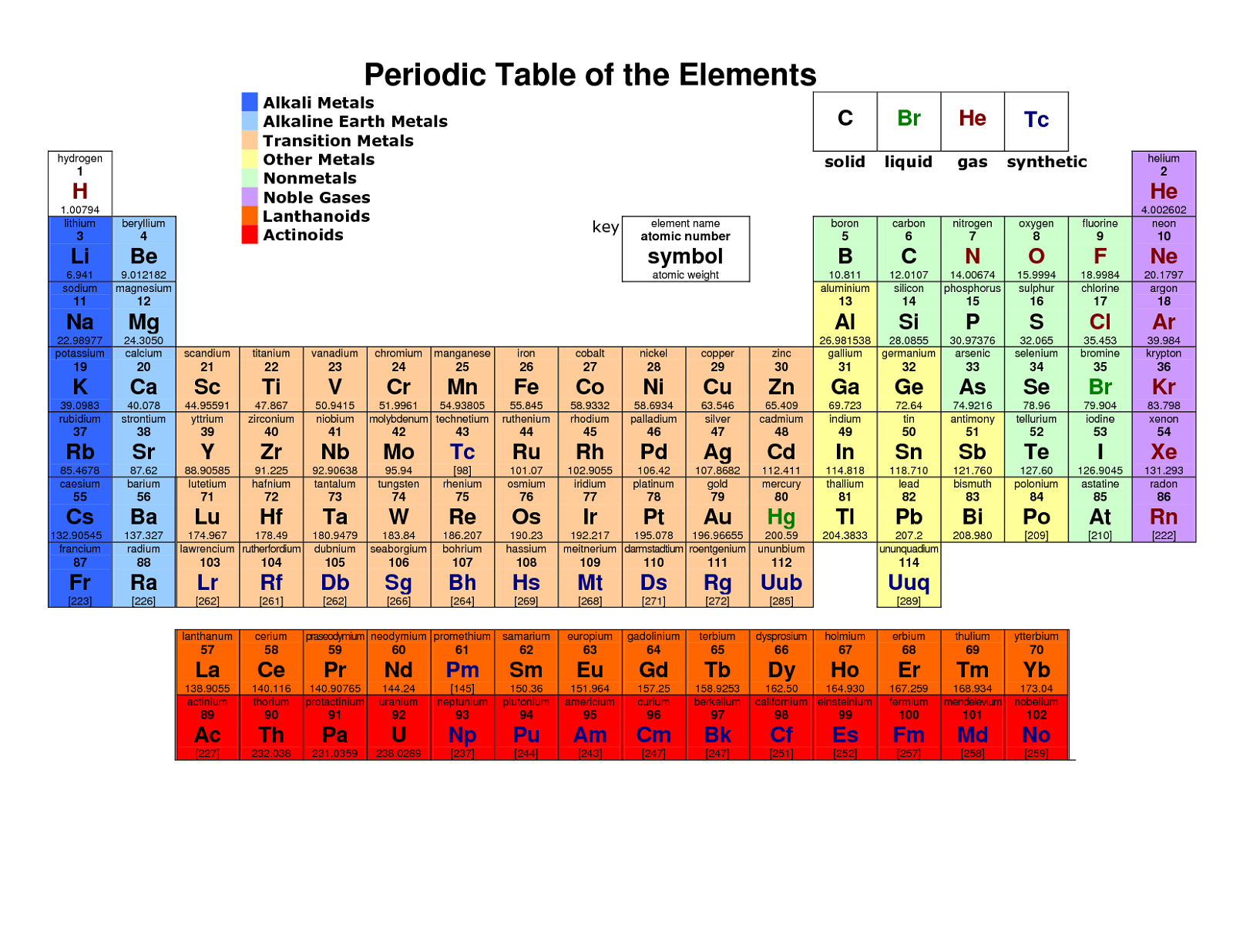
Understanding the Periodic Table: A Comprehensive Guide for Students
The periodic table is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number and are grouped into rows called periods and columns called groups or families. The periodic table is a powerful tool for chemists and other scientists, allowing them to predict the properties and behavior of elements.
History of the Periodic Table
The periodic table was first developed by Dmitri Mendeleev in 1869. Mendeleev, a Russian chemist, noticed that when the elements were arranged in order of increasing atomic weight, certain patterns emerged. He realized that elements with similar properties recurred at regular intervals, and he used this information to create the first periodic table.
Structure of the Periodic Table
The periodic table is divided into several sections:
- Metals: Located on the left side and in the center of the periodic table, metals are typically shiny, malleable, and good conductors of electricity.
- Nonmetals: Located on the right side of the periodic table, nonmetals are typically dull, brittle, and poor conductors of electricity.
- Metalloids: Located on the border between metals and nonmetals, metalloids exhibit some properties of both.
- Noble Gases: Located in the far right column of the periodic table, noble gases are unreactive and do not typically form compounds with other elements.
- Halogen Family: Located in the far right column of the periodic table, the halogen family includes elements that readily form ions with a charge of -1.
- Alkali Metal Family: Located in the far left column of the periodic table, the alkali metal family includes elements that readily form ions with a charge of +1.
Periodic Trends
The periodic table exhibits several trends, including:
- Atomic Radius: The size of an atom decreases as you move from left to right across a period, and increases as you move down a group.
- Electronegativity: The ability of an atom to attract electrons decreases as you move down a group, and increases as you move from left to right across a period.
- Ionization Energy: The energy required to remove an electron from an atom decreases as you move down a group, and increases as you move from left to right across a period.
Types of Elements
The periodic table includes several types of elements, including:
- Alkali Metals: Group 1 elements, which readily form ions with a charge of +1.
- Alkaline Earth Metals: Group 2 elements, which readily form ions with a charge of +2.
- Halogens: Group 17 elements, which readily form ions with a charge of -1.
- Noble Gases: Group 18 elements, which do not typically form compounds with other elements.
Printable Periodic Table Worksheet
Here is a printable periodic table worksheet for students:
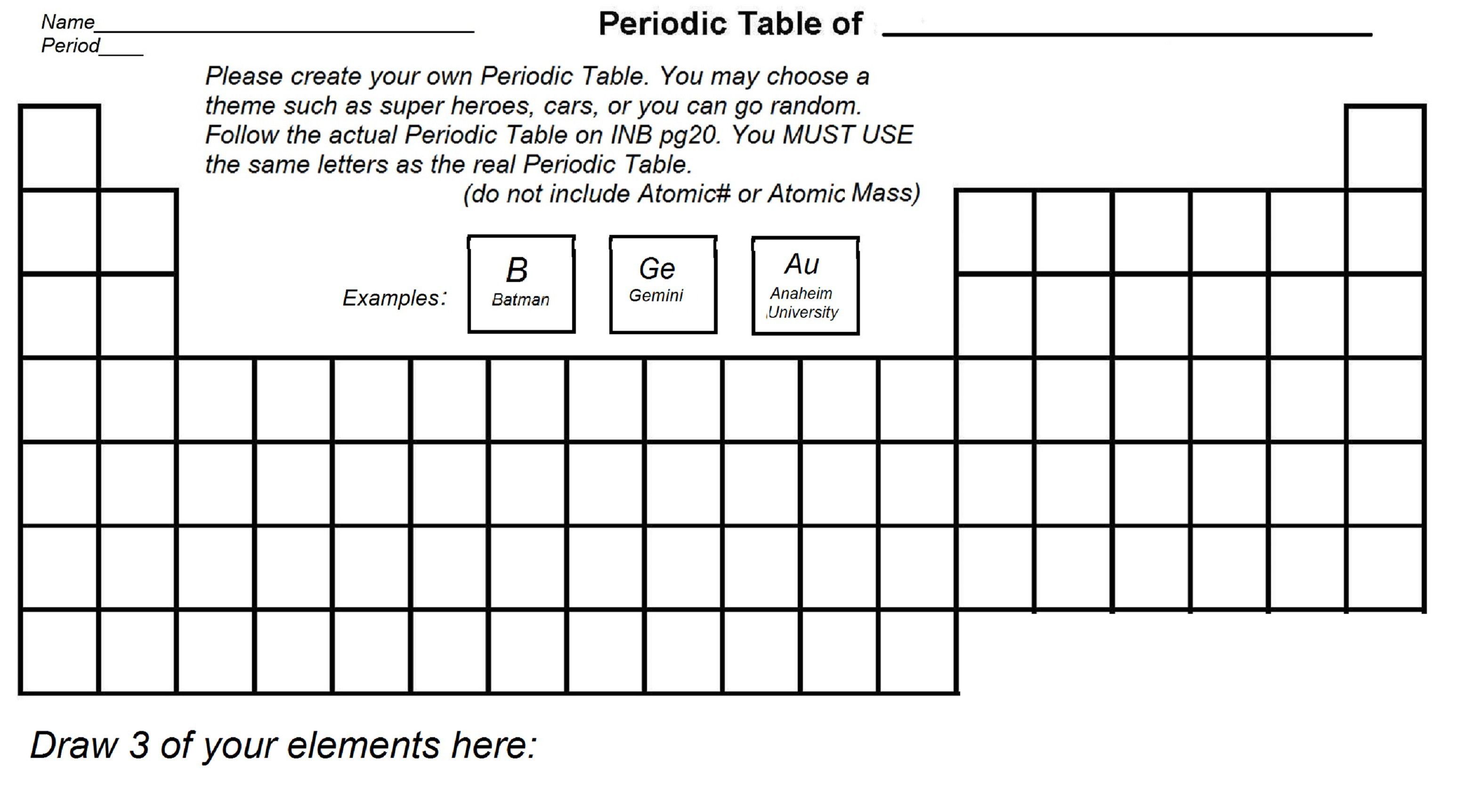
| Element | Symbol | Atomic Number | Atomic Mass |
|---|---|---|---|
| Hydrogen | H | 1 | 1.00794 |
| Helium | He | 2 | 4.002602 |
| Lithium | Li | 3 | 6.941 |
| Beryllium | Be | 4 | 9.012182 |
| Boron | B | 5 | 10.811 |
| Carbon | C | 6 | 12.0107 |
| Nitrogen | N | 7 | 14.0067 |
| Oxygen | O | 8 | 15.9994 |
| Fluorine | F | 9 | 18.998403163 |
| Neon | Ne | 10 | 20.1797 |
| Sodium | Na | 11 | 22.98976928 |
| Magnesium | Mg | 12 | 24.305 |
| Aluminum | Al | 13 | 26.9815385 |
| Silicon | Si | 14 | 28.0855 |
| Phosphorus | P | 15 | 30.973761998 |
| Sulfur | S | 16 | 32.064 |
| Chlorine | Cl | 17 | 35.453 |
| Argon | Ar | 18 | 39.9483 |
Using the Periodic Table Worksheet
Here are some ways you can use the periodic table worksheet:
- Identify Elements: Use the worksheet to identify elements by their symbol, atomic number, and atomic mass.
- Determine Element Type: Use the worksheet to determine whether an element is a metal, nonmetal, or metalloid.
- Predict Element Properties: Use the worksheet to predict the properties of an element, such as its atomic radius, electronegativity, and ionization energy.
📝 Note: The periodic table worksheet is a useful tool for students to learn about the elements and their properties. However, it is not a substitute for hands-on learning and experimentation.
Conclusion
The periodic table is a powerful tool for chemists and other scientists, allowing them to predict the properties and behavior of elements. By understanding the structure and trends of the periodic table, students can gain a deeper understanding of the elements and their properties. The printable periodic table worksheet provided in this article is a useful tool for students to learn about the elements and their properties.
What is the periodic table?
+The periodic table is a tabular display of the known chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties.
What is the structure of the periodic table?
+The periodic table is divided into several sections, including metals, nonmetals, metalloids, noble gases, halogen family, and alkali metal family.
What are some of the trends of the periodic table?
+Some of the trends of the periodic table include atomic radius, electronegativity, and ionization energy.
Related Terms:
- Periodic Table Worksheet pdf
- Periodic table worksheet answers