Chemical Reaction Products Prediction Worksheet Answers
Understanding Chemical Reaction Products Prediction
Chemical reactions involve the transformation of one or more substances into new substances. Predicting the products of a chemical reaction is crucial in chemistry, as it helps in understanding the reaction’s outcome and the properties of the resulting substances. In this worksheet, we will explore the answers to a chemical reaction products prediction exercise, focusing on various types of reactions and the principles behind predicting their products.
Types of Chemical Reactions
Chemical reactions can be broadly classified into several types, including:
- Synthesis reactions: Two or more substances combine to form a new compound.
- Decomposition reactions: A single compound breaks down into two or more simpler substances.
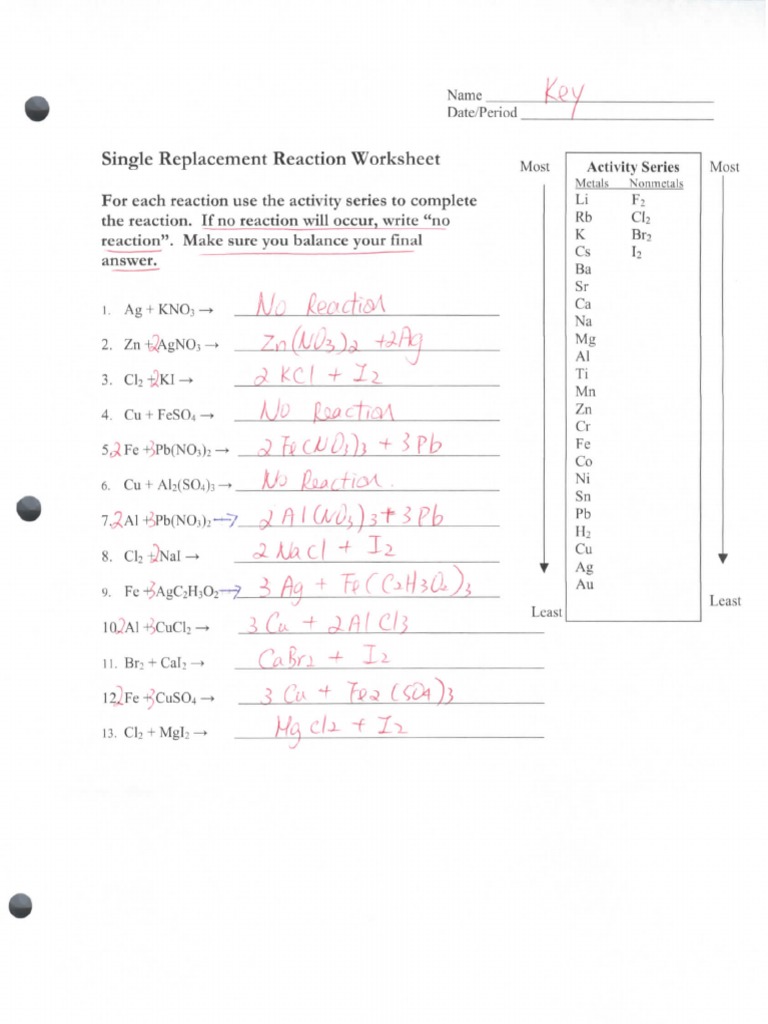
- Replacement reactions: One element replaces another element in a compound.
- Combustion reactions: A substance reacts with oxygen to produce heat and light.
Chemical Reaction Products Prediction Worksheet Answers
The following are answers to a chemical reaction products prediction worksheet, covering various types of reactions:
Synthesis Reactions

| Reaction | Products |
|---|---|
| 2Na + Cl2 → | 2NaCl |
| CaO + H2O → | Ca(OH)2 |
| 2H2 + O2 → | 2H2O |
Decomposition Reactions
| Reaction | Products |
|---|---|
| 2H2O → | 2H2 + O2 |
| CaCO3 → | CaO + CO2 |
| 2KClO3 → | 2KCl + 3O2 |
Replacement Reactions
| Reaction | Products |
|---|---|
| Zn + CuSO4 → | ZnSO4 + Cu |
| Mg + 2HCl → | MgCl2 + H2 |
| Fe + Cu(NO3)2 → | Fe(NO3)2 + Cu |
Combustion Reactions
| Reaction | Products |
|---|---|
| CH4 + 2O2 → | CO2 + 2H2O |
| 2C2H6 + 7O2 → | 4CO2 + 6H2O |
| C3H8 + 5O2 → | 3CO2 + 4H2O |
🔍 Note: These reactions assume complete combustion, where all the fuel is burned and all the oxygen is consumed.
Acid-Base Reactions
| Reaction | Products |
|---|---|
| HCl + NaOH → | NaCl + H2O |
| H2SO4 + 2KOH → | K2SO4 + 2H2O |
| HNO3 + Ca(OH)2 → | Ca(NO3)2 + 2H2O |
Redox Reactions
| Reaction | Products |
|---|---|
| 2Al + Fe2O3 → | Al2O3 + 2Fe |
| Cu + 2AgNO3 → | Cu(NO3)2 + 2Ag |
| 2Na + Cl2 → | 2NaCl |
💡 Note: Redox reactions involve the transfer of electrons from one substance to another, resulting in a change in oxidation states.
Conclusion
Predicting the products of chemical reactions requires a deep understanding of the reaction types and the principles behind them. By analyzing the reactants and applying the relevant reaction principles, we can accurately predict the products of various chemical reactions. This exercise has demonstrated the application of these principles to different types of reactions, providing a solid foundation for understanding chemical reaction products prediction.
What are the main types of chemical reactions?
+The main types of chemical reactions are synthesis, decomposition, replacement, and combustion reactions.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction involves the combination of two or more substances to form a new compound, while a decomposition reaction involves the breakdown of a single compound into two or more simpler substances.
What is the importance of predicting chemical reaction products?
+Predicting chemical reaction products is crucial in understanding the reaction’s outcome and the properties of the resulting substances, which is essential in various fields such as chemistry, biology, and engineering.