5 Ways to Balance Chemical Equations Easily
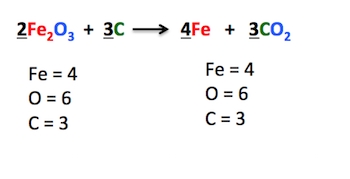
Understanding the Importance of Balancing Chemical Equations
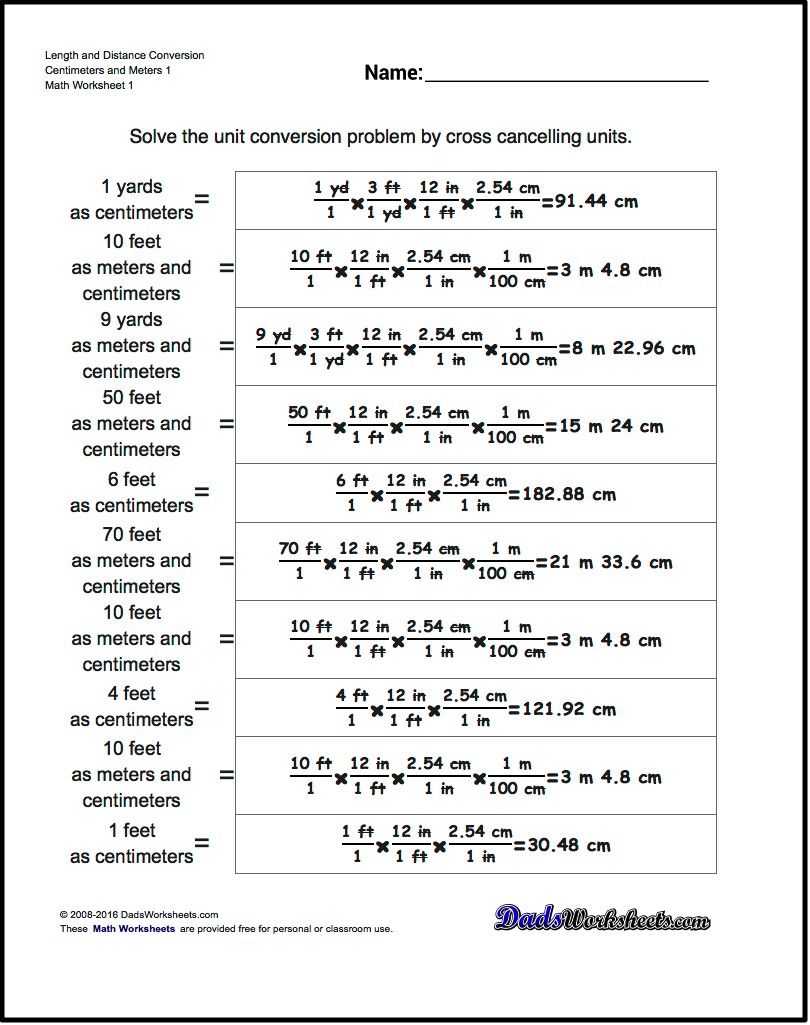
Balancing chemical equations is a crucial step in chemistry, as it ensures that the number of atoms for each element is the same on both the reactant and product sides. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Unbalanced equations can lead to incorrect conclusions and calculations, making it essential to master the art of balancing chemical equations. In this article, we will explore five ways to balance chemical equations easily.
Method 1: Trial and Error
The trial and error method involves adjusting the coefficients (numbers in front of the formulas of reactants or products) to balance the equation. This method is simple and straightforward but can be time-consuming and may not always lead to the correct solution. To use this method:
- Write down the unbalanced equation.
- Identify the elements that are not balanced.
- Adjust the coefficients of the reactants or products to balance the equation.
- Check if the equation is balanced by counting the number of atoms of each element on both sides.
🚨 Note: This method can be tedious and may not always lead to the correct solution, especially for complex equations.
Method 2: Balancing by Inspection
The balancing by inspection method involves looking at the equation and identifying the elements that are not balanced. This method requires a good understanding of chemistry and the ability to visualize the reaction. To use this method:
- Write down the unbalanced equation.
- Identify the elements that are not balanced.
- Look for elements that appear only once on each side of the equation.
- Balance those elements first.
- Continue balancing the remaining elements.
👍 Note: This method is faster and more efficient than the trial and error method but requires a good understanding of chemistry.
Method 3: The Half-Reaction Method
The half-reaction method involves splitting the equation into two half-reactions: oxidation and reduction. This method is useful for complex equations and redox reactions. To use this method:
- Write down the unbalanced equation.
- Split the equation into two half-reactions: oxidation and reduction.
- Balance each half-reaction separately.
- Combine the balanced half-reactions to get the final balanced equation.
💡 Note: This method is useful for complex equations and redox reactions but requires a good understanding of oxidation and reduction reactions.
Method 4: The Matrix Method
The matrix method involves creating a matrix of the coefficients of the reactants and products. This method is useful for complex equations with multiple reactants and products. To use this method:
- Write down the unbalanced equation.
- Create a matrix of the coefficients of the reactants and products.
- Use the matrix to identify the elements that are not balanced.
- Adjust the coefficients to balance the equation.
📊 Note: This method is useful for complex equations with multiple reactants and products but requires a good understanding of matrices.
Method 5: Using Online Tools
There are many online tools available that can help balance chemical equations. These tools can save time and effort, especially for complex equations. To use online tools:
- Search for online chemical equation balancers.
- Enter the unbalanced equation into the tool.
- The tool will generate the balanced equation.
🔍 Note: Online tools can save time and effort but may not always provide the correct solution. It's essential to check the solution manually.
The five methods discussed above can help balance chemical equations easily. It’s essential to practice and master the methods to become proficient in balancing chemical equations.
What is the law of conservation of mass?
+The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
Why is balancing chemical equations important?
+Balancing chemical equations ensures that the number of atoms for each element is the same on both the reactant and product sides, which is essential for accurate calculations and conclusions.
What is the best method for balancing chemical equations?
+The best method for balancing chemical equations depends on the complexity of the equation and personal preference. The five methods discussed above can be used to balance chemical equations easily.