Matter's Physical Properties Worksheet for Students

Understanding Matter's Physical Properties: A Comprehensive Guide for Students
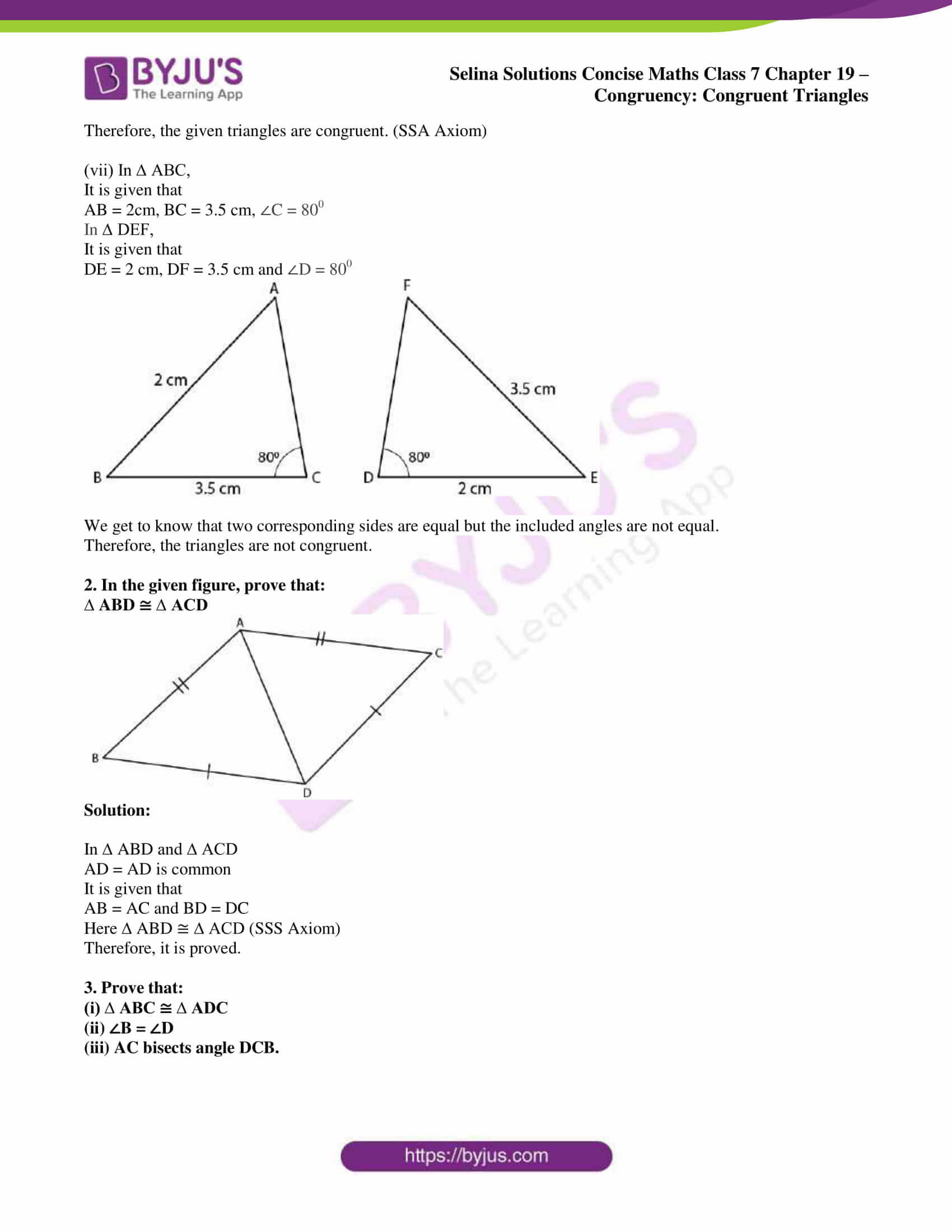
Matter is the substance that makes up all physical objects in the universe. It can exist in various forms, such as solids, liquids, and gases, and has several physical properties that can be observed and measured. In this article, we will delve into the different types of physical properties of matter, their definitions, and examples, as well as provide a worksheet for students to practice and reinforce their understanding.
What are Physical Properties of Matter?
Physical properties of matter are characteristics that can be observed or measured without changing the composition of the substance. These properties include:
- Color: The appearance of an object when light is reflected off its surface.
- Odor: The smell of a substance.
- Taste: The sensation of a substance in the mouth.
- Texture: The feel of a substance when touched.
- Melting Point: The temperature at which a solid changes state to become a liquid.
- Boiling Point: The temperature at which a liquid changes state to become a gas.
- Density: The amount of mass per unit volume of a substance.
Types of Physical Properties
There are two main categories of physical properties: intensive and extensive properties.
- Intensive Properties: These properties do not depend on the amount of matter present. Examples include:
- Melting point
- Boiling point
- Density
- Color
- Extensive Properties: These properties depend on the amount of matter present. Examples include:
- Mass
- Volume
- Length
Examples of Physical Properties
Here are some examples of physical properties:
- The color of a red apple is a physical property.
- The melting point of ice is 0°C, which is a physical property.
- The density of water is 1 gram per milliliter, which is a physical property.
- The texture of a rough stone is a physical property.
Worksheet: Physical Properties of Matter
Complete the following table by identifying whether each property is intensive or extensive:
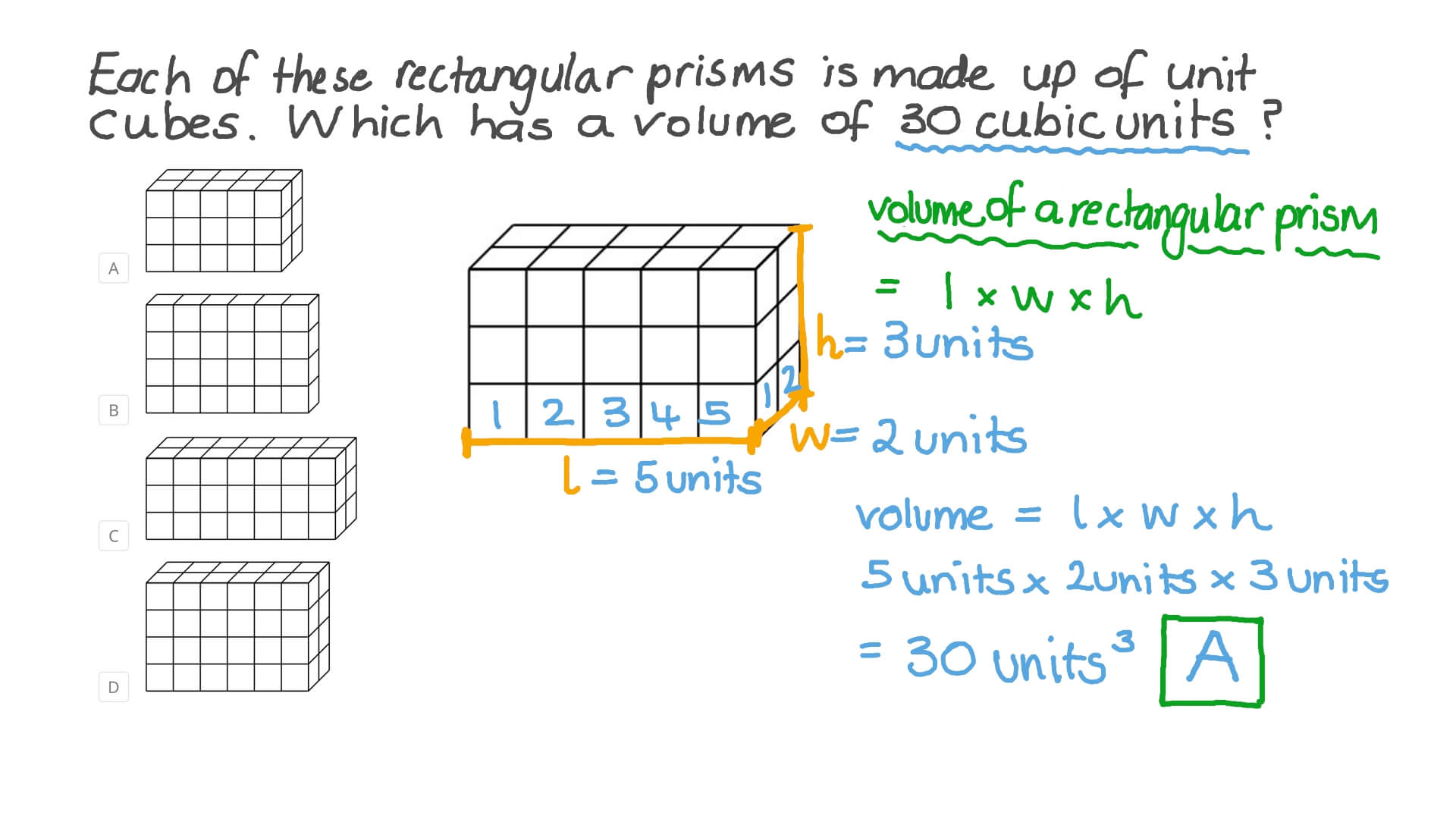
| Property | Intensive or Extensive |
|---|---|
| Mass | _____________ |
| Density | _____________ |
| Color | _____________ |
| Volume | _____________ |
| Boiling Point | _____________ |
Answer Key:
- Mass: Extensive
- Density: Intensive
- Color: Intensive
- Volume: Extensive
- Boiling Point: Intensive
Notes
📝 Note: Physical properties can be used to identify and distinguish between different substances.
📝 Note: Changes in physical properties can indicate changes in the state of matter, such as melting or boiling.
The ability to understand and identify physical properties of matter is crucial in various fields of science, including chemistry and physics. By practicing with the worksheet provided, students can reinforce their knowledge and develop a deeper understanding of the physical properties of matter.
What is the difference between intensive and extensive properties?
+Intensive properties do not depend on the amount of matter present, while extensive properties depend on the amount of matter present.
What is an example of an intensive physical property?
+Density is an example of an intensive physical property, as it does not depend on the amount of matter present.
What is the importance of understanding physical properties of matter?
+Understanding physical properties of matter is crucial in various fields of science, including chemistry and physics, as it allows us to identify and distinguish between different substances and understand changes in their state.
Related Terms:
- teacher synergy llc
- Khan Academy
- IXL
- BrainPOP
- Udacity
- Duolingo