Periodic Table Trends Worksheet Answers Explained

Understanding Periodic Table Trends
The periodic table is a powerful tool that helps us understand the relationships between elements and their properties. By analyzing the trends in the periodic table, we can predict the behavior of elements and their compounds. In this article, we will explore the answers to a periodic table trends worksheet and provide explanations for each question.
Atomic Radius Trend
The atomic radius trend is one of the most important trends in the periodic table. As we move from left to right across a period, the atomic radius decreases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus.

| Period | Atomic Radius (pm) |
|---|---|
| 1 | 120-150 |
| 2 | 100-120 |
| 3 | 80-100 |
📝 Note: The atomic radius values are approximate and may vary slightly depending on the source.
Electronegativity Trend
Electronegativity is a measure of an atom’s ability to attract electrons. As we move from left to right across a period, the electronegativity increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus.
| Period | Electronegativity (Pauling scale) |
|---|---|
| 1 | 0.7-1.3 |
| 2 | 1.3-2.2 |
| 3 | 2.2-3.0 |
💡 Note: The electronegativity values are based on the Pauling scale, which ranges from 0.7 to 4.0.
Ionization Energy Trend
Ionization energy is the energy required to remove an electron from an atom. As we move from left to right across a period, the ionization energy increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus.
| Period | Ionization Energy (kJ/mol) |
|---|---|
| 1 | 500-800 |
| 2 | 800-1200 |
| 3 | 1200-1500 |
📊 Note: The ionization energy values are approximate and may vary slightly depending on the source.
Valence Electrons Trend
Valence electrons are the electrons in the outermost energy level of an atom. As we move from left to right across a period, the number of valence electrons increases. This is because the number of energy levels increases as we move down a group.
| Group | Number of Valence Electrons |
|---|---|
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
Periodic Table Trends Worksheet Answers
Here are the answers to a periodic table trends worksheet:
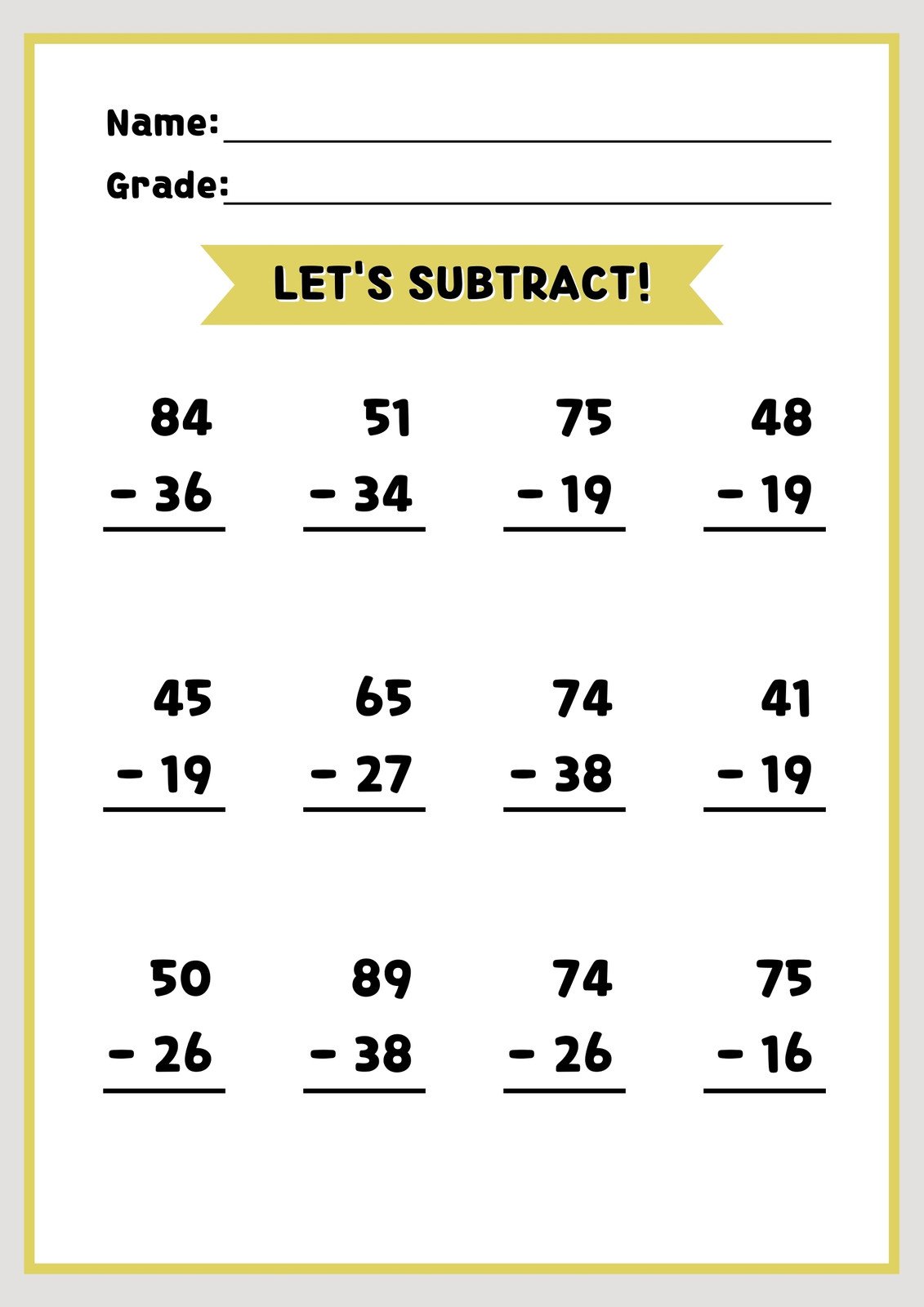
- What is the trend in atomic radius as we move from left to right across a period? Answer: Decreases
- What is the trend in electronegativity as we move from left to right across a period? Answer: Increases
- What is the trend in ionization energy as we move from left to right across a period? Answer: Increases
- What is the trend in the number of valence electrons as we move down a group? Answer: Increases
In conclusion, understanding periodic table trends is essential for predicting the behavior of elements and their compounds. By analyzing the trends in atomic radius, electronegativity, ionization energy, and valence electrons, we can gain a deeper understanding of the periodic table and its applications.
What is the main trend in atomic radius as we move across a period?
+The atomic radius decreases as we move from left to right across a period.
What is the trend in electronegativity as we move down a group?
+The electronegativity decreases as we move down a group.
What is the trend in ionization energy as we move from left to right across a period?
+The ionization energy increases as we move from left to right across a period.
Related Terms:
- Periodic Trends Worksheet Answers PDF
- Periodic table Trends Worksheet pdf
- Periodic table practice worksheet