5 Essential Periodic Table Trends to Know
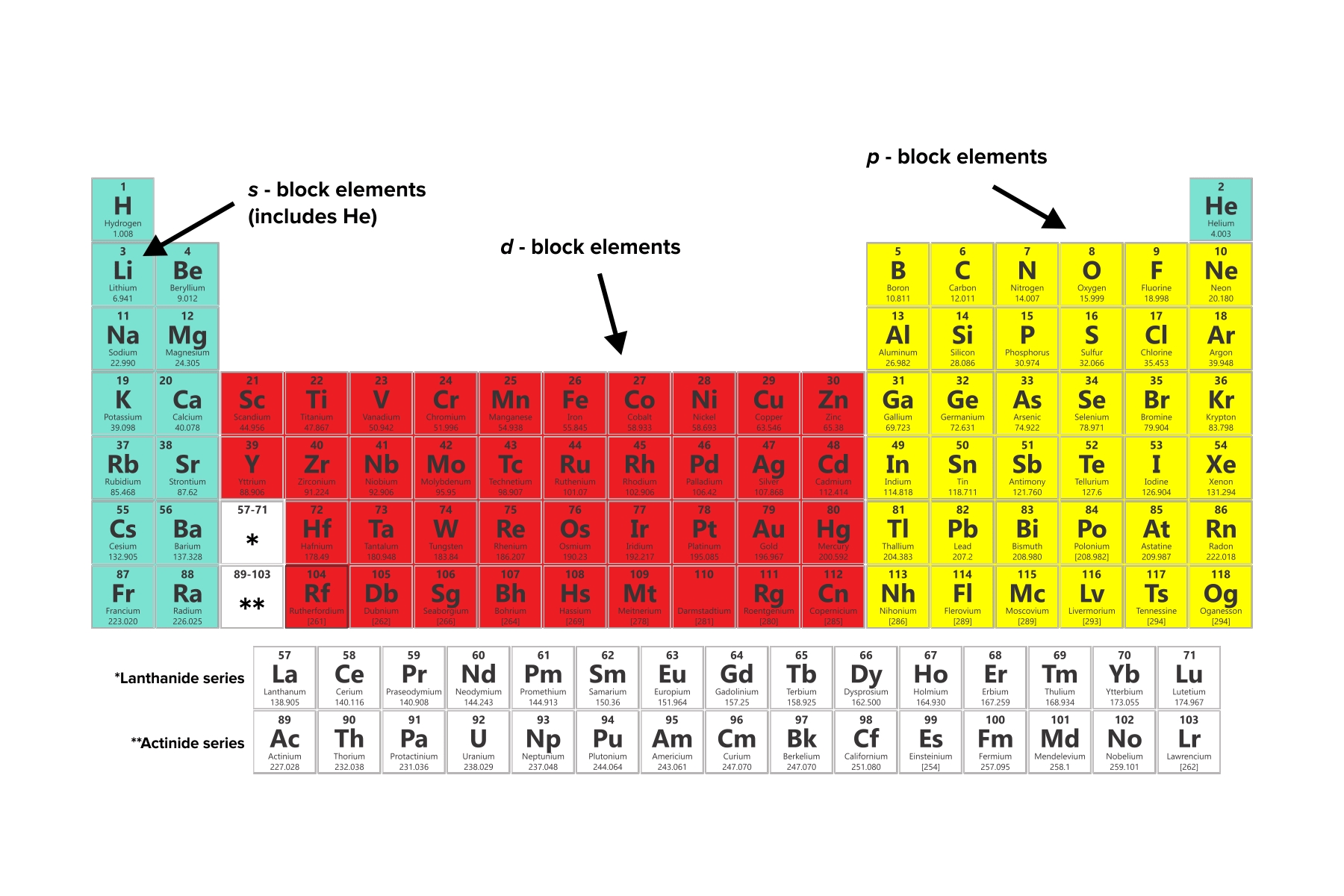
Understanding Periodic Table Trends
The periodic table is a powerful tool for chemists and scientists, allowing them to organize and understand the properties of elements. By analyzing trends in the periodic table, we can gain insights into the behavior of elements and make predictions about their properties. In this article, we will explore five essential periodic table trends to know.
Trend 1: Atomic Radius
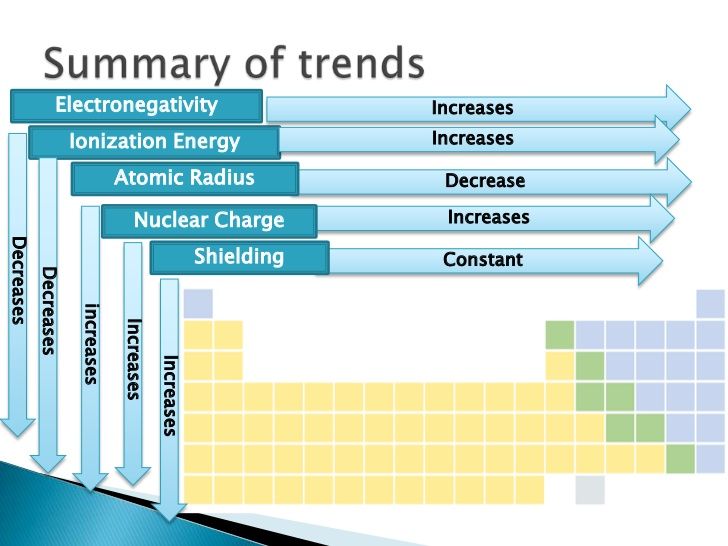
One of the most fundamental trends in the periodic table is the atomic radius trend. As we move from left to right across a period, the atomic radius of elements decreases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
Key Points:
- Atomic radius decreases across a period (left to right)
- Atomic radius increases down a group (top to bottom)
| Group | Atomic Radius (pm) |
|---|---|
| 1 (Alkali Metals) | 200-300 |
| 2 (Alkaline Earth Metals) | 150-250 |
| 13 (Boron Group) | 100-200 |
| 14 (Carbon Group) | 80-150 |
📝 Note: Atomic radius values are approximate and can vary depending on the source.
Trend 2: Electronegativity
Electronegativity is a measure of an element’s ability to attract electrons in a covalent bond. As we move from left to right across a period, the electronegativity of elements increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus, resulting in a greater attraction for electrons.
Key Points:
- Electronegativity increases across a period (left to right)
- Electronegativity decreases down a group (top to bottom)
| Group | Electronegativity (Pauling Scale) |
|---|---|
| 1 (Alkali Metals) | 0.7-1.3 |
| 2 (Alkaline Earth Metals) | 1.2-1.7 |
| 13 (Boron Group) | 2.0-2.5 |
| 14 (Carbon Group) | 2.5-3.0 |
📝 Note: Electronegativity values can vary depending on the source and method used to calculate them.
Trend 3: Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. As we move from left to right across a period, the ionization energy of elements increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus, resulting in a greater energy required to remove an electron.
Key Points:
- Ionization energy increases across a period (left to right)
- Ionization energy decreases down a group (top to bottom)
| Group | Ionization Energy (kJ/mol) |
|---|---|
| 1 (Alkali Metals) | 400-600 |
| 2 (Alkaline Earth Metals) | 600-800 |
| 13 (Boron Group) | 800-1000 |
| 14 (Carbon Group) | 1000-1200 |
📝 Note: Ionization energy values can vary depending on the source and method used to calculate them.
Trend 4: Electron Affinity
Electron affinity is the energy released when an electron is added to an atom. As we move from left to right across a period, the electron affinity of elements increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus, resulting in a greater energy released when an electron is added.
Key Points:
- Electron affinity increases across a period (left to right)
- Electron affinity decreases down a group (top to bottom)
| Group | Electron Affinity (kJ/mol) |
|---|---|
| 1 (Alkali Metals) | 50-100 |
| 2 (Alkaline Earth Metals) | 100-200 |
| 13 (Boron Group) | 200-300 |
| 14 (Carbon Group) | 300-400 |
📝 Note: Electron affinity values can vary depending on the source and method used to calculate them.
Trend 5: Metallic Character
Metallic character refers to the ability of an element to exhibit the properties of a metal, such as conductivity and malleability. As we move from left to right across a period, the metallic character of elements decreases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus, resulting in a lower ability to conduct electricity and exhibit other metallic properties.
Key Points:
- Metallic character decreases across a period (left to right)
- Metallic character increases down a group (top to bottom)
| Group | Metallic Character |
|---|---|
| 1 (Alkali Metals) | High |
| 2 (Alkaline Earth Metals) | Medium |
| 13 (Boron Group) | Low |
| 14 (Carbon Group) | Nonmetallic |
📝 Note: Metallic character is a qualitative trend and can vary depending on the source and method used to define it.
In conclusion, understanding the trends in the periodic table is crucial for making predictions about the properties of elements and their behavior in chemical reactions. By analyzing the trends in atomic radius, electronegativity, ionization energy, electron affinity, and metallic character, we can gain a deeper understanding of the periodic table and its applications.
What is the most important trend in the periodic table?
+The most important trend in the periodic table is the atomic radius trend, which decreases across a period and increases down a group.
How do electronegativity and ionization energy relate to each other?
+Electronegativity and ionization energy are both related to the attraction of electrons to the nucleus. Electronegativity is a measure of an element’s ability to attract electrons in a covalent bond, while ionization energy is the energy required to remove an electron from an atom.
What is the difference between electron affinity and ionization energy?
+Electron affinity is the energy released when an electron is added to an atom, while ionization energy is the energy required to remove an electron from an atom.