Calculating Success: Percent Yield Worksheet Made Easy
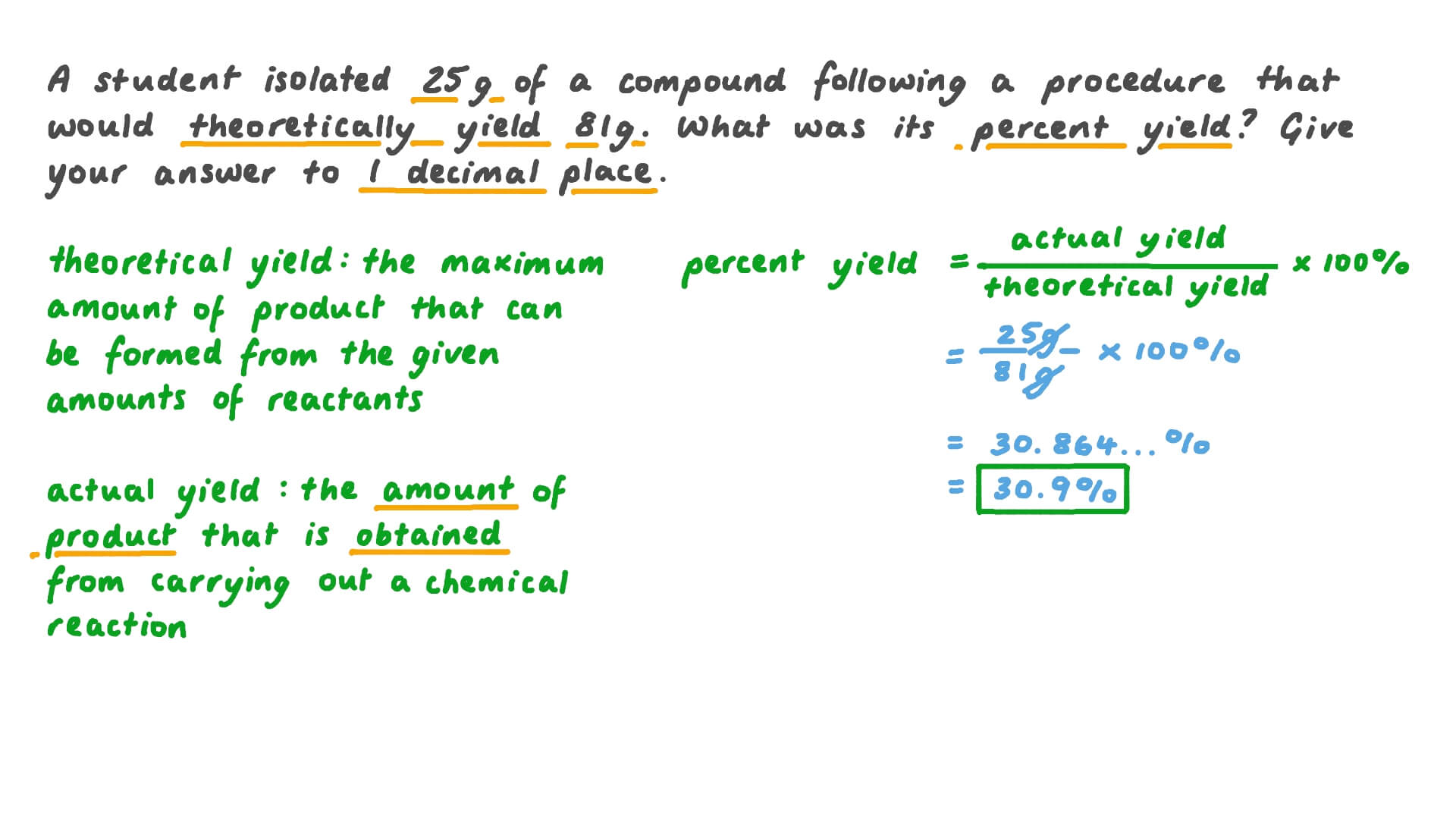
Understanding Percent Yield: A Comprehensive Guide
Calculating percent yield is a crucial concept in chemistry, and it can be a bit tricky to grasp at first. However, with the right approach and tools, it can become second nature. In this article, we will break down the concept of percent yield, provide a step-by-step guide on how to calculate it, and offer a simple worksheet to help you practice.
What is Percent Yield?
Percent yield is a measure of the efficiency of a chemical reaction. It is calculated by comparing the actual yield of a product to the theoretical yield. Theoretical yield is the maximum amount of product that can be obtained from a reaction, based on the stoichiometry of the reactants. Actual yield, on the other hand, is the amount of product that is actually obtained.
Why is Percent Yield Important?
Percent yield is important because it helps chemists and researchers to:
- Evaluate the efficiency of a reaction
- Identify potential problems or areas for improvement
- Compare the results of different experiments or reactions
- Scale up reactions to larger quantities
How to Calculate Percent Yield
Calculating percent yield is a straightforward process that involves the following steps:
- Determine the theoretical yield of the reaction.
- Measure the actual yield of the reaction.
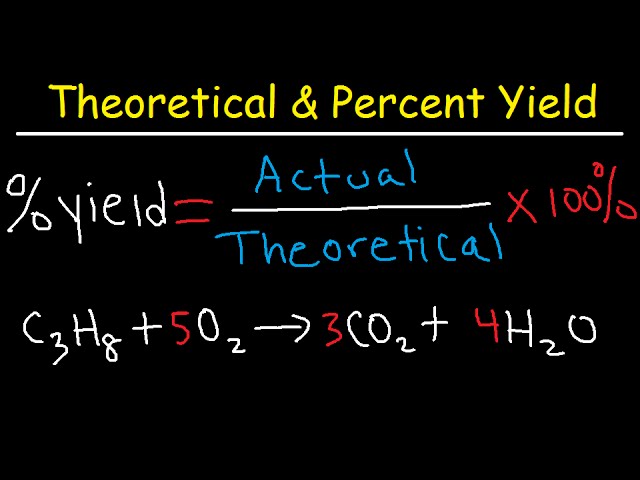
- Calculate the percent yield using the following formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100
📝 Note: Make sure to express the actual and theoretical yields in the same units, such as grams or moles.
Example Problem
Suppose we have a reaction that produces 25 grams of product, and the theoretical yield is 30 grams. What is the percent yield?
- Determine the theoretical yield: 30 grams
- Measure the actual yield: 25 grams
- Calculate the percent yield:
Percent Yield = (25 g / 30 g) x 100 = 83.3%
Worksheet: Calculating Percent Yield
Here is a simple worksheet to help you practice calculating percent yield:
| Reaction | Theoretical Yield (g) | Actual Yield (g) | Percent Yield |
|---|---|---|---|
| 1 | 20 | 18 | ? |
| 2 | 50 | 45 | ? |
| 3 | 100 | 90 | ? |
| 4 | 25 | 22 | ? |
| 5 | 75 | 65 | ? |
📝 Note: Try to calculate the percent yield for each reaction on your own before checking the answers below.
| Reaction | Theoretical Yield (g) | Actual Yield (g) | Percent Yield |
|---|---|---|---|
| 1 | 20 | 18 | 90% |
| 2 | 50 | 45 | 90% |
| 3 | 100 | 90 | 90% |
| 4 | 25 | 22 | 88% |
| 5 | 75 | 65 | 86.7% |
Tips and Tricks
Here are some tips and tricks to help you master the concept of percent yield:
- Make sure to express the actual and theoretical yields in the same units.
- Double-check your calculations to avoid errors.
- Use a calculator to simplify the calculations.
- Practice, practice, practice! The more you practice, the more comfortable you will become with calculating percent yield.
What is the difference between actual yield and theoretical yield?
+Theoretical yield is the maximum amount of product that can be obtained from a reaction, based on the stoichiometry of the reactants. Actual yield, on the other hand, is the amount of product that is actually obtained.
Why is percent yield important in chemistry?
+Percent yield is important because it helps chemists and researchers to evaluate the efficiency of a reaction, identify potential problems or areas for improvement, and compare the results of different experiments or reactions.
How do I calculate percent yield?
+To calculate percent yield, you need to determine the theoretical yield of the reaction, measure the actual yield, and then use the formula: Percent Yield = (Actual Yield / Theoretical Yield) x 100.
By following these steps and practicing with the worksheet, you should be able to master the concept of percent yield and become proficient in calculating it. Remember to always double-check your calculations and express the actual and theoretical yields in the same units. With practice and patience, you will become more comfortable and confident in your ability to calculate percent yield.