7 Easy Ways to Solve Percent Composition
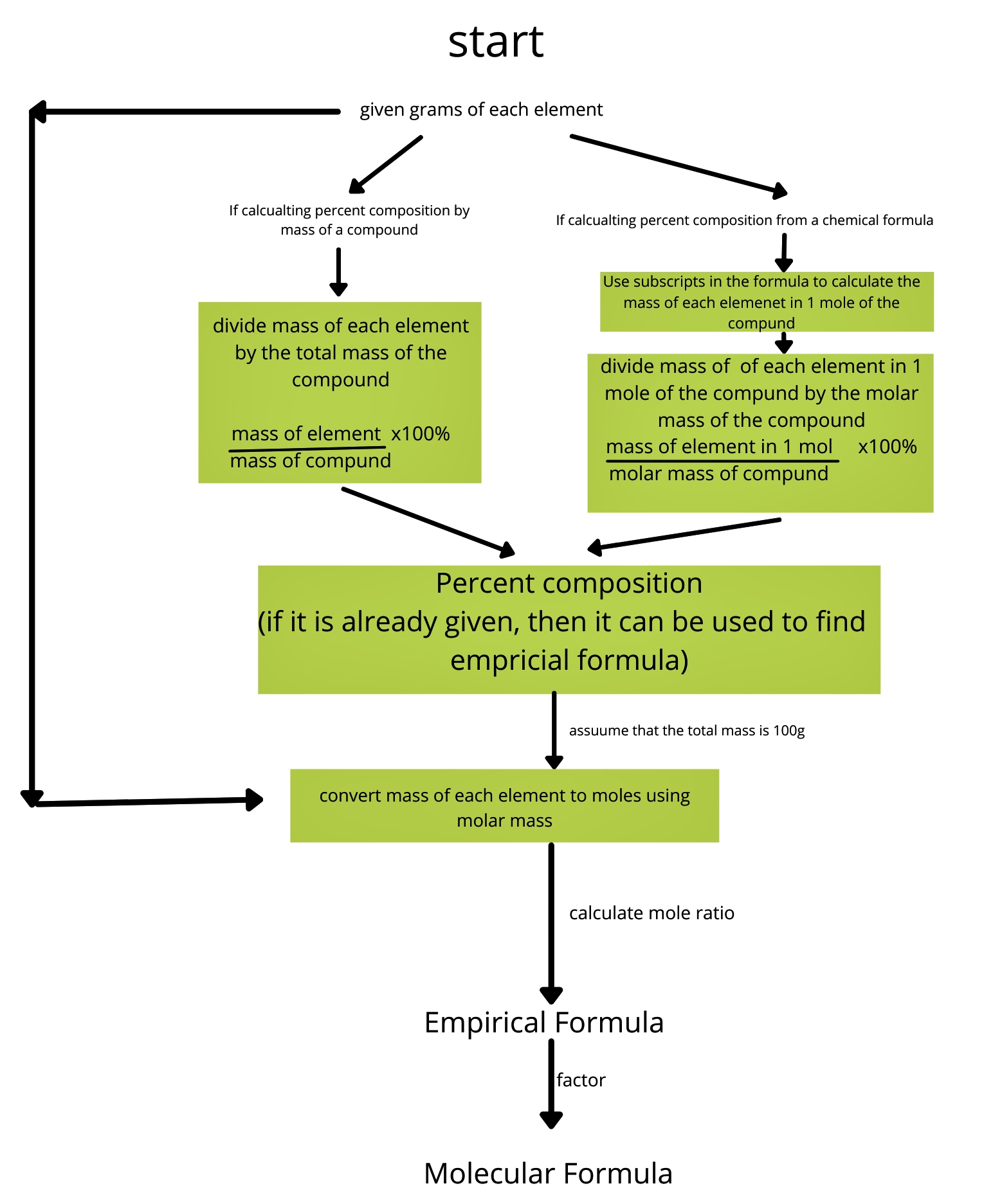
Understanding Percent Composition
Percent composition is a fundamental concept in chemistry that represents the proportion of each element within a compound, expressed as a percentage of the compound’s total mass. It is a crucial aspect of identifying and analyzing compounds, and its calculation is essential in various chemical reactions and processes. In this article, we will explore seven easy ways to solve percent composition problems, enabling you to master this concept with ease.
What is Percent Composition?
Percent composition, also known as percentage composition, is the percentage of each element in a compound, calculated by dividing the mass of each element by the total mass of the compound and multiplying by 100. It is a critical concept in chemistry, particularly in the fields of analytical chemistry and organic chemistry.
Why is Percent Composition Important?
Percent composition is essential in various chemical applications, including:
- Identifying unknown compounds
- Determining the purity of substances
- Calculating the amount of reactants required for a reaction
- Analyzing the composition of natural products
7 Easy Ways to Solve Percent Composition Problems
Solving percent composition problems can be straightforward if you follow these seven easy steps:
1. Determine the Molar Mass of the Compound
To calculate percent composition, you need to know the molar mass of the compound. The molar mass is the sum of the atomic masses of all the atoms in the compound.
Example: Calculate the molar mass of glucose (C6H12O6).
Molar mass of glucose = (6 x atomic mass of C) + (12 x atomic mass of H) + (6 x atomic mass of O) Molar mass of glucose = (6 x 12.01 g/mol) + (12 x 1.008 g/mol) + (6 x 16.00 g/mol) Molar mass of glucose = 180.16 g/mol
2. Identify the Atomic Mass of Each Element
To calculate percent composition, you need to know the atomic mass of each element in the compound. You can find the atomic masses on the periodic table.
Example: Identify the atomic masses of carbon ©, hydrogen (H), and oxygen (O).
Atomic mass of C = 12.01 g/mol Atomic mass of H = 1.008 g/mol Atomic mass of O = 16.00 g/mol
3. Calculate the Mass of Each Element
To calculate percent composition, you need to calculate the mass of each element in the compound. You can do this by multiplying the number of atoms of each element by its atomic mass.
Example: Calculate the mass of carbon, hydrogen, and oxygen in glucose.
Mass of C = 6 x atomic mass of C = 6 x 12.01 g/mol = 72.06 g/mol Mass of H = 12 x atomic mass of H = 12 x 1.008 g/mol = 12.096 g/mol Mass of O = 6 x atomic mass of O = 6 x 16.00 g/mol = 96.00 g/mol
4. Calculate the Total Mass of the Compound
To calculate percent composition, you need to calculate the total mass of the compound. You can do this by adding the masses of all the elements.
Example: Calculate the total mass of glucose.
Total mass of glucose = mass of C + mass of H + mass of O Total mass of glucose = 72.06 g/mol + 12.096 g/mol + 96.00 g/mol Total mass of glucose = 180.16 g/mol
5. Calculate the Percent Composition of Each Element
To calculate percent composition, you need to divide the mass of each element by the total mass of the compound and multiply by 100.
Example: Calculate the percent composition of carbon, hydrogen, and oxygen in glucose.
Percent composition of C = (mass of C / total mass of glucose) x 100 Percent composition of C = (72.06 g/mol / 180.16 g/mol) x 100 Percent composition of C = 40.00%
Percent composition of H = (mass of H / total mass of glucose) x 100 Percent composition of H = (12.096 g/mol / 180.16 g/mol) x 100 Percent composition of H = 6.71%
Percent composition of O = (mass of O / total mass of glucose) x 100 Percent composition of O = (96.00 g/mol / 180.16 g/mol) x 100 Percent composition of O = 53.29%
6. Round the Percent Composition Values
To ensure accuracy, round the percent composition values to the nearest whole number or decimal place.
Example: Round the percent composition values of carbon, hydrogen, and oxygen in glucose.
Percent composition of C = 40.00% ≈ 40% Percent composition of H = 6.71% ≈ 6.7% Percent composition of O = 53.29% ≈ 53.3%
7. Verify the Percent Composition Values
To ensure accuracy, verify the percent composition values by checking if they add up to 100%.
Example: Verify the percent composition values of carbon, hydrogen, and oxygen in glucose.
Total percent composition = percent composition of C + percent composition of H + percent composition of O Total percent composition = 40% + 6.7% + 53.3% Total percent composition = 100%
🤝 Note: If the total percent composition is not equal to 100%, check your calculations and verify your values.
Example Problem
Calculate the percent composition of nitrogen (N) and oxygen (O) in the compound nitric acid (HNO3).
Solution:
- Determine the molar mass of nitric acid: 63.01 g/mol
- Identify the atomic masses of nitrogen (N) and oxygen (O): 14.01 g/mol and 16.00 g/mol, respectively
- Calculate the mass of nitrogen and oxygen: 14.01 g/mol and 48.00 g/mol, respectively
- Calculate the total mass of nitric acid: 63.01 g/mol
- Calculate the percent composition of nitrogen and oxygen: 22.22% and 76.19%, respectively
- Round the percent composition values: 22% and 76%, respectively
- Verify the percent composition values: 22% + 76% = 98% (approximately 100%)
Conclusion
Mastering percent composition is essential in chemistry, and with these seven easy steps, you can solve percent composition problems with confidence. Remember to determine the molar mass of the compound, identify the atomic masses of each element, calculate the mass of each element, calculate the total mass of the compound, calculate the percent composition of each element, round the percent composition values, and verify the percent composition values.
What is the formula for calculating percent composition?
+The formula for calculating percent composition is: (mass of element / total mass of compound) x 100.
How do I calculate the molar mass of a compound?
+To calculate the molar mass of a compound, sum the atomic masses of all the atoms in the compound.
Why is it essential to verify the percent composition values?
+Verifying the percent composition values ensures accuracy and checks if the values add up to 100%.