Mastering Percent Composition Made Easy
Understanding Percent Composition: A Comprehensive Guide
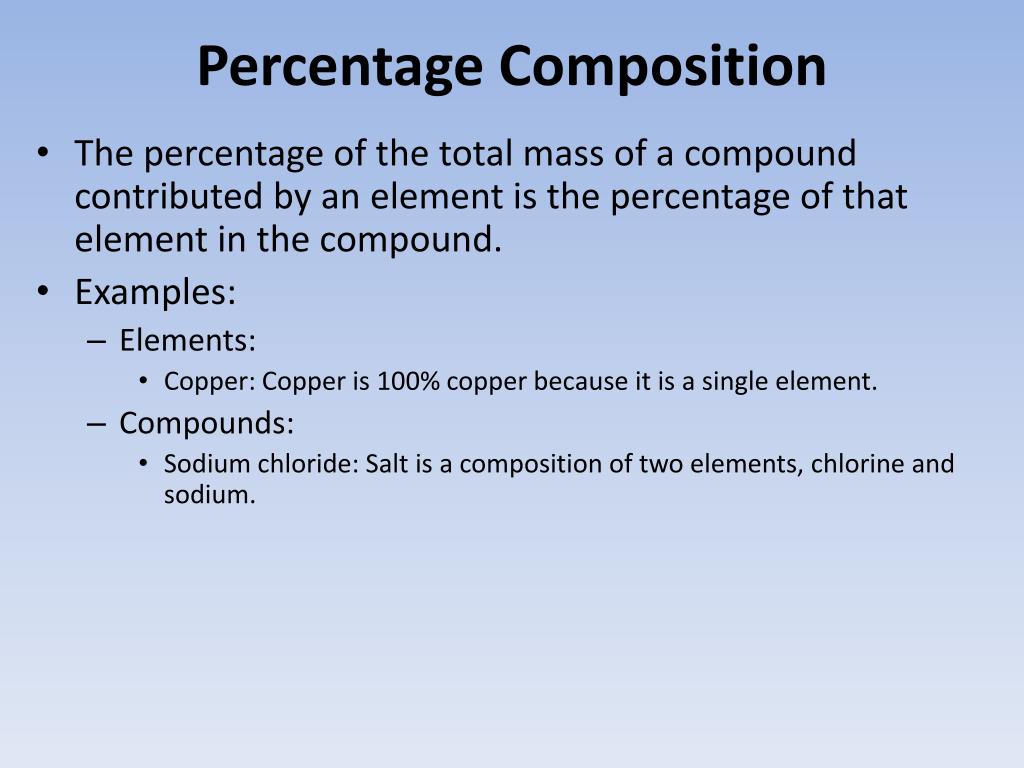
Percent composition is a fundamental concept in chemistry that deals with the proportion of each element within a compound. It is a crucial aspect of understanding the properties and behavior of chemical substances. In this article, we will delve into the world of percent composition, exploring its definition, significance, and application. By the end of this comprehensive guide, you will be well-equipped to calculate and understand percent composition with ease.
What is Percent Composition?
Percent composition refers to the percentage of each element present in a compound by mass. It is calculated by dividing the mass of each element by the total mass of the compound and multiplying by 100. The resulting value represents the proportion of each element in the compound.
Why is Percent Composition Important?
Percent composition is vital in various aspects of chemistry, including:
- Chemical analysis: Percent composition helps identify the composition of unknown substances.
- Chemical synthesis: It ensures the correct proportions of reactants are used to produce the desired compound.
- Materials science: Percent composition affects the physical and chemical properties of materials.
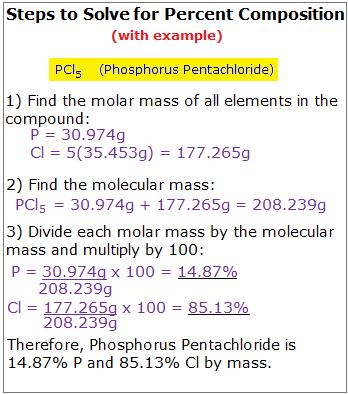
Calculating Percent Composition: A Step-by-Step Guide
Calculating percent composition is a straightforward process that involves the following steps:
- Determine the molecular formula: Identify the molecular formula of the compound.
- Calculate the molar mass: Calculate the molar mass of the compound.
- Determine the mass of each element: Calculate the mass of each element present in the compound.
- Calculate the percent composition: Divide the mass of each element by the total mass of the compound and multiply by 100.
🔍 Note: Make sure to use the correct units and rounding when performing calculations.
Example Calculation: Percent Composition of Water (H2O)
Let’s calculate the percent composition of water (H2O):
- Molecular formula: H2O
- Molar mass: 18.02 g/mol
- Mass of hydrogen: 2 x 1.01 g/mol = 2.02 g/mol
- Mass of oxygen: 16.00 g/mol
- Percent composition:
- Hydrogen: (2.02 g/mol / 18.02 g/mol) x 100 = 11.19%
- Oxygen: (16.00 g/mol / 18.02 g/mol) x 100 = 88.81%
| Element | Mass (g/mol) | Percent Composition |
|---|---|---|
| Hydrogen | 2.02 | 11.19% |
| Oxygen | 16.00 | 88.81% |
Empirical Formula from Percent Composition: A Step-by-Step Guide
An empirical formula can be determined from percent composition by following these steps:
- Assume a 100g sample: Assume a 100g sample of the compound.
- Calculate the mass of each element: Calculate the mass of each element present in the sample.
- Convert mass to moles: Convert the mass of each element to moles.
- Determine the simplest whole-number ratio: Determine the simplest whole-number ratio of moles.
📝 Note: Make sure to round numbers to the nearest whole number when determining the simplest ratio.
Example Calculation: Empirical Formula from Percent Composition
Let’s calculate the empirical formula of a compound with the following percent composition:
- Carbon: 40.00%
- Hydrogen: 6.67%
- Oxygen: 53.33%
- Assume a 100g sample: Assume a 100g sample of the compound.
- Calculate the mass of each element:
- Carbon: 40.00 g
- Hydrogen: 6.67 g
- Oxygen: 53.33 g
- Convert mass to moles:
- Carbon: 40.00 g / 12.01 g/mol = 3.33 mol
- Hydrogen: 6.67 g / 1.01 g/mol = 6.61 mol
- Oxygen: 53.33 g / 16.00 g/mol = 3.33 mol
- Determine the simplest whole-number ratio:
- Carbon: 3.33 mol / 3.33 = 1
- Hydrogen: 6.61 mol / 3.33 = 2
- Oxygen: 3.33 mol / 3.33 = 1
Empirical formula: CH2O
By following these steps and understanding the concept of percent composition, you can easily calculate and determine the empirical formula of a compound.
In conclusion, percent composition is a fundamental concept in chemistry that deals with the proportion of each element within a compound. By understanding how to calculate percent composition and empirical formulas, you can better comprehend the properties and behavior of chemical substances. With practice and patience, you will become proficient in calculating percent composition and applying it to real-world problems.
What is the difference between empirical and molecular formulas?
+Empirical formulas represent the simplest whole-number ratio of atoms, while molecular formulas represent the actual number of atoms in a molecule.
How do I calculate the molar mass of a compound?
+The molar mass of a compound is calculated by summing the atomic masses of each element present in the compound.
What is the significance of percent composition in chemistry?
+Percent composition is significant in chemistry as it helps identify the composition of unknown substances, ensures the correct proportions of reactants are used to produce the desired compound, and affects the physical and chemical properties of materials.