Chemical Equation Worksheet: Mastering Reaction Parts
Understanding Chemical Equations: A Comprehensive Guide
Chemical equations are a crucial part of chemistry, representing the reactions that occur between substances. They provide a concise way to show the reactants, products, and sometimes the reaction conditions or catalysts involved. Mastering chemical equations is essential for understanding various chemical processes, from simple reactions to complex industrial applications.
What is a Chemical Equation?
A chemical equation is a symbolic representation of a chemical reaction. It uses chemical formulas to represent the reactants and products, along with other necessary information such as coefficients (numbers in front of formulas of reactants or products), and sometimes symbols for catalysts, heat, or light. The equation must follow the law of conservation of mass, meaning that the number of atoms for each element is the same on both the reactant and product sides.
Parts of a Chemical Equation
Understanding the parts of a chemical equation is crucial for interpreting and writing them correctly.
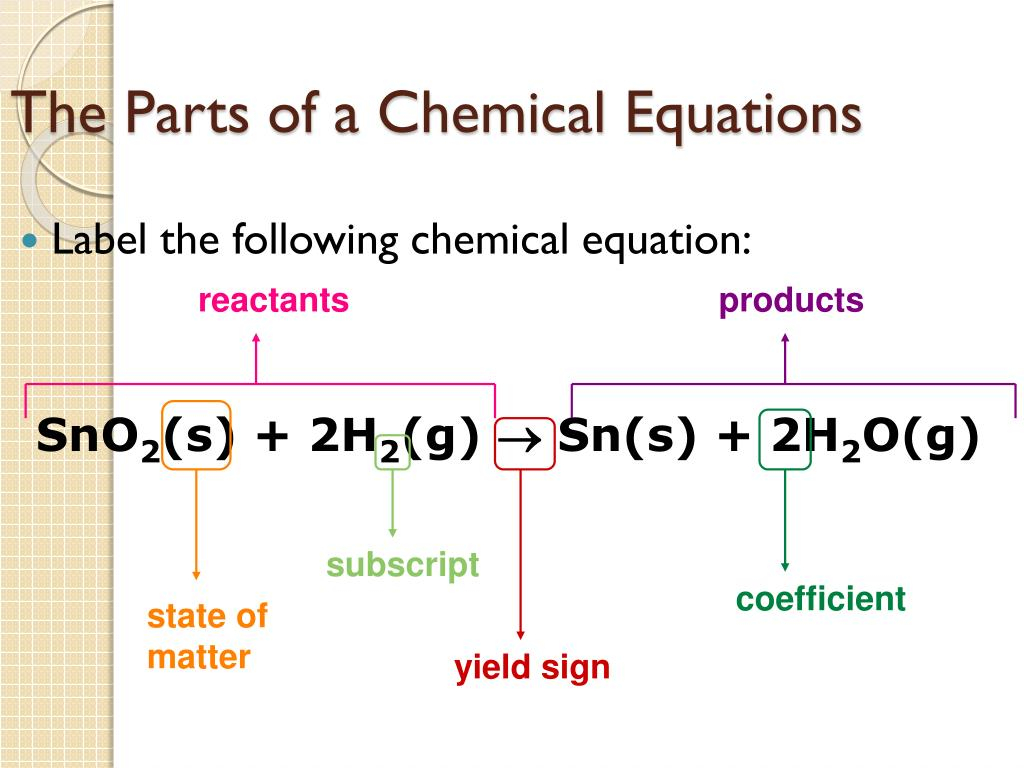
- Reactants: These are the substances that undergo change in a chemical reaction. They are written on the left side of the equation.
- Products: These are the substances formed as a result of the chemical reaction. They are written on the right side of the equation.
- Coefficients: These are numbers placed in front of the formulas of reactants or products to balance the equation.
- Subscripts: These are small numbers written to the right of the chemical symbol of an element within a formula. They indicate the number of atoms of that element in the compound.
- States of Matter: Symbols are used to indicate the state of matter of each substance: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous solution.
- Arrow: The arrow (→) indicates the direction of the reaction. A single arrow indicates a forward reaction, while a double arrow (↔) indicates an equilibrium reaction.
- Catalyst: Sometimes, a substance is added to speed up the reaction without being consumed in the process. This is indicated above or below the arrow.
Writing Chemical Equations
Writing a chemical equation involves several steps:
- Write the Unbalanced Equation: Start by writing the reactants on the left and the products on the right, using their chemical formulas.
- Balance the Equation: Balance the equation by adding coefficients (numbers in front of the formulas) so that the number of atoms for each element is the same on both sides.
- Check the Balance: Verify that each element has the same number of atoms on both the reactant and product sides.
- Add States of Matter: Include symbols for the states of matter of the reactants and products.
- Include Catalysts or Conditions: Add symbols or words to indicate any catalysts or special conditions (like heat or light) required for the reaction.
💡 Note: It's essential to understand that balancing chemical equations requires patience and practice. It involves trial and error, as well as a good understanding of chemistry concepts.
Types of Chemical Equations
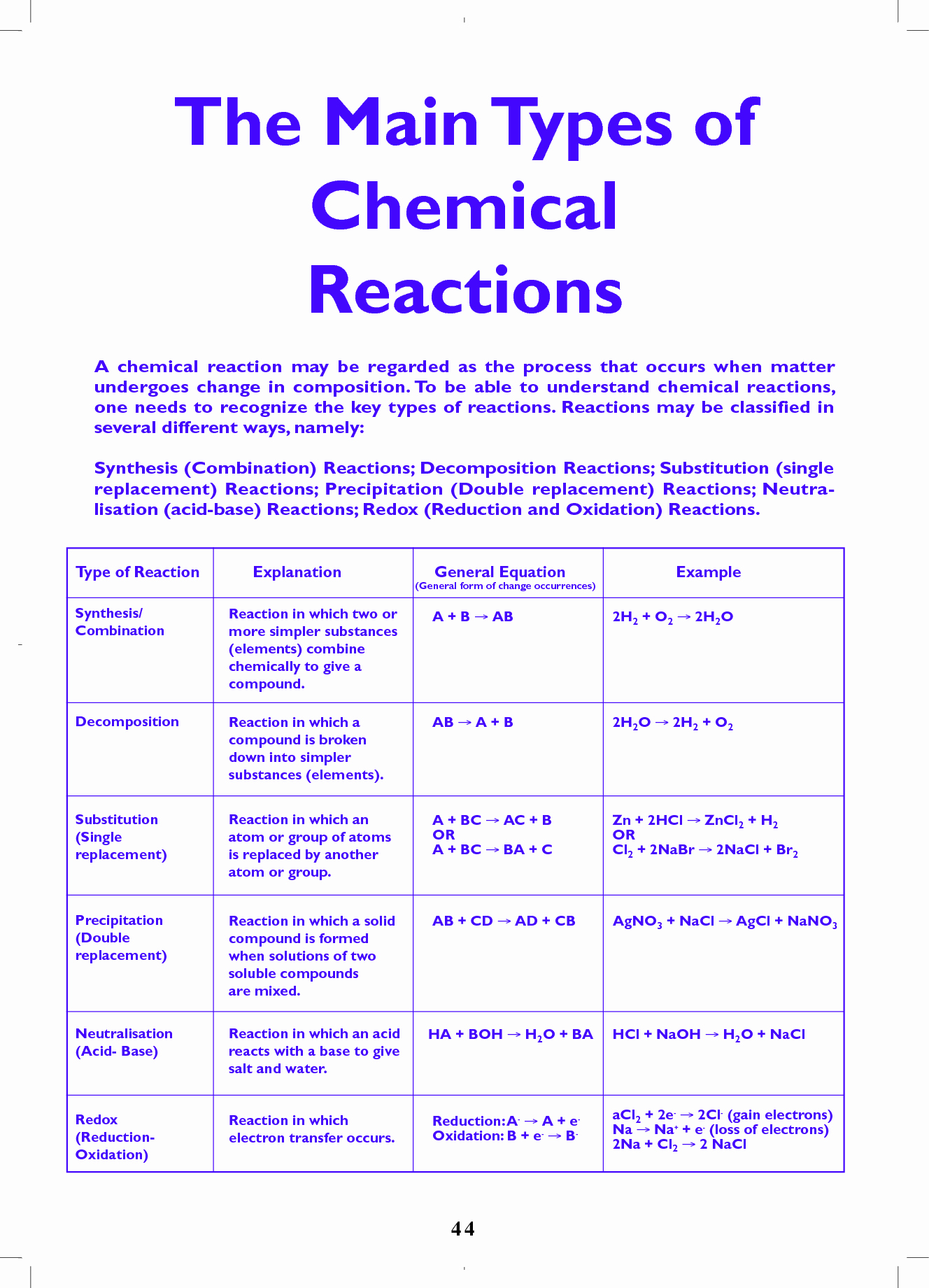
Chemical equations can be classified based on the type of reaction they represent:
- Synthesis (Combination) Reaction: Two or more substances combine to form a single product.
- Decomposition Reaction: A single compound breaks down into two or more substances.
- Replacement (Single Displacement) Reaction: One element displaces another element in a compound.
- Double Displacement (Metathesis) Reaction: Two compounds exchange partners, resulting in two new compounds.
- Combustion Reaction: A substance reacts with oxygen to produce heat and light, often producing carbon dioxide and water as well.
Importance of Chemical Equations
Chemical equations play a vital role in various fields:
- Chemical Industry: They are used to design and optimize processes for manufacturing chemicals and materials.
- Environmental Science: Understanding chemical reactions helps in addressing environmental issues like pollution and climate change.
- Biological Systems: Chemical equations are essential for understanding metabolic pathways and biochemical processes.
- Research and Development: They are used to propose and test hypotheses in scientific research.
Tools for Working with Chemical Equations
Several tools and techniques are available for working with chemical equations:
- Chemical Equation Balancers: Online tools that can automatically balance chemical equations.
- Periodic Table: A tabular display of the elements, organized by their atomic number, electron configuration, and recurring chemical properties.
- Chemistry Software: Programs like ChemDraw and MarvinSketch that can help in drawing and manipulating chemical structures and equations.
| Tool | Description |
|---|---|
| Chemical Equation Balancers | Automatically balance chemical equations. |
| Periodic Table | Organizes elements by their atomic number and chemical properties. |
| Chemistry Software | Draws and manipulates chemical structures and equations. |
To summarize, chemical equations are a fundamental part of chemistry that represent chemical reactions in a concise way. Understanding their parts, how to write them, and the types of chemical equations is crucial for mastering chemistry. With practice and the right tools, one can become proficient in working with chemical equations, which is essential for various applications in chemistry, environmental science, biological systems, and research and development.
What is the purpose of coefficients in a chemical equation?
+Coefficients are numbers placed in front of the formulas of reactants or products to balance the equation, ensuring that the number of atoms for each element is the same on both sides.
What types of reactions are represented by chemical equations?
+Chemical equations can represent various types of reactions, including synthesis, decomposition, replacement, double displacement, and combustion reactions.
Why is it important to balance chemical equations?
+Balancing chemical equations is crucial for ensuring that the number of atoms for each element is conserved, which is a fundamental principle of chemistry.