Master Oxidation Numbers with 10 Easy Worksheet Answers
Oxidation numbers are a crucial concept in chemistry, particularly in understanding the behavior of elements in different compounds. Mastering oxidation numbers is essential for any student of chemistry, and one of the best ways to achieve this is through practice. In this article, we’ll provide you with a comprehensive guide on how to determine oxidation numbers, followed by 10 easy worksheet answers to help you practice.
What are Oxidation Numbers?
Oxidation numbers, also known as oxidation states, are a way of keeping track of the transfer of electrons in a chemical reaction. They are assigned to atoms in a compound to indicate the degree of oxidation or reduction. Oxidation numbers are useful in identifying the type of chemical reaction that has occurred, such as oxidation or reduction.
Rules for Assigning Oxidation Numbers
To assign oxidation numbers, follow these simple rules:
- Free elements: The oxidation number of a free element is always 0. For example, the oxidation number of oxygen (O) in its elemental form is 0.
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of sodium (Na+) is +1, while the oxidation number of chloride (Cl-) is -1.
- Oxygen: The oxidation number of oxygen is usually -2, except in peroxides where it is -1.
- Hydrogen: The oxidation number of hydrogen is usually +1, except in hydrides where it is -1.
- Fluorine: The oxidation number of fluorine is always -1.
Determining Oxidation Numbers in Compounds
To determine the oxidation number of an atom in a compound, follow these steps:
- Write the formula of the compound.
- Identify the atoms in the compound.
- Assign oxidation numbers to each atom using the rules above.
- Check if the total oxidation number of the compound is equal to its charge.
🔍 Note: The total oxidation number of a compound is equal to its charge. For example, the total oxidation number of sodium chloride (NaCl) is 0, since it is a neutral compound.
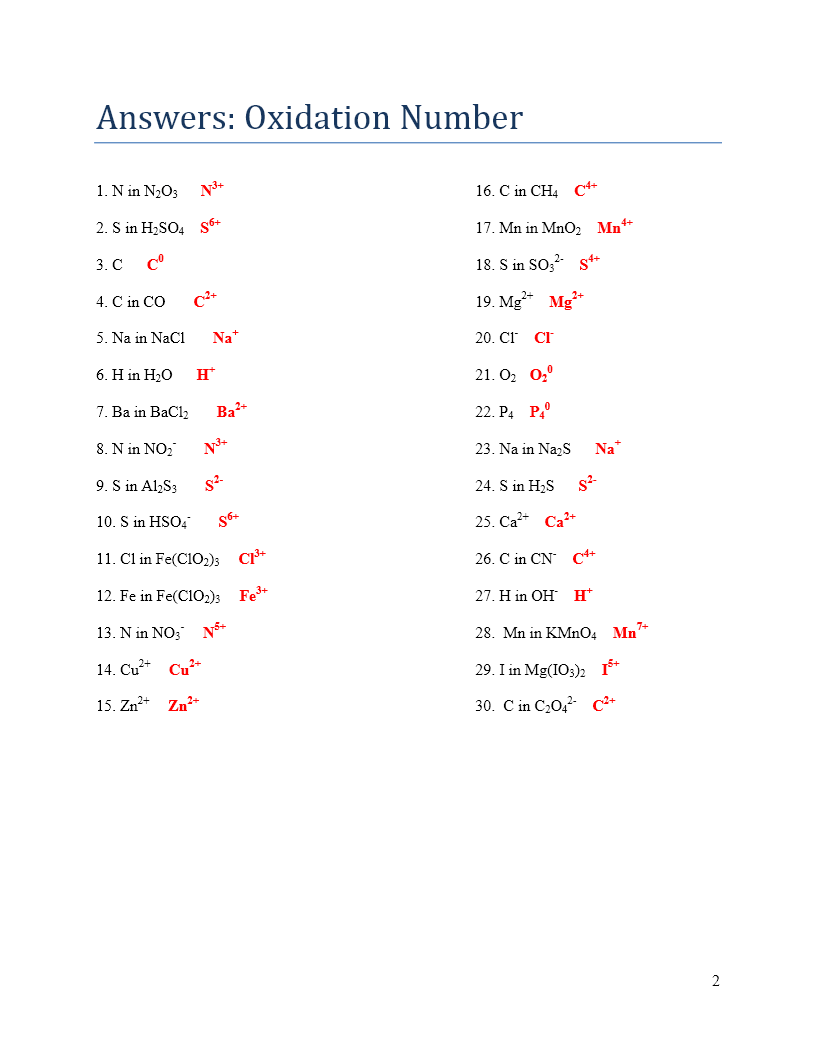
Worksheet Answers
Here are 10 easy worksheet answers to help you practice determining oxidation numbers:
What is the oxidation number of oxygen in water (H2O)? Answer: -2
What is the oxidation number of sodium in sodium chloride (NaCl)? Answer: +1
What is the oxidation number of hydrogen in hydrogen peroxide (H2O2)? Answer: +1
What is the oxidation number of fluorine in sodium fluoride (NaF)? Answer: -1
What is the oxidation number of carbon in carbon dioxide (CO2)? Answer: +4
What is the oxidation number of oxygen in potassium nitrate (KNO3)? Answer: -2
What is the oxidation number of hydrogen in hydrochloric acid (HCl)? Answer: +1
What is the oxidation number of nitrogen in ammonia (NH3)? Answer: -3
What is the oxidation number of sulfur in sulfuric acid (H2SO4)? Answer: +6
What is the oxidation number of oxygen in potassium permanganate (KMnO4)? Answer: -2
Tips for Mastering Oxidation Numbers
Here are some tips to help you master oxidation numbers:
- Practice, practice, practice! The more you practice, the more familiar you’ll become with assigning oxidation numbers.
- Use flashcards to help you memorize the rules for assigning oxidation numbers.
- Start with simple compounds and work your way up to more complex ones.
- Check your answers with a partner or tutor to ensure you’re getting them correct.
Mastering oxidation numbers takes time and practice, but with these tips and worksheet answers, you’ll be well on your way to becoming a pro!
What is the purpose of oxidation numbers in chemistry?
+Oxidation numbers help us keep track of the transfer of electrons in a chemical reaction, which is essential for understanding the behavior of elements in different compounds.
How do I determine the oxidation number of an atom in a compound?
+To determine the oxidation number of an atom in a compound, write the formula of the compound, identify the atoms, assign oxidation numbers using the rules, and check if the total oxidation number is equal to the charge of the compound.
What is the oxidation number of oxygen in peroxides?
+The oxidation number of oxygen in peroxides is -1, which is an exception to the usual -2 rule.
Related Terms:
- Finding oxidation numbers worksheet 2
- Oxidation number Worksheet doc
- Worksheet oxidation numbers
- Oxidation numbers rules
- Assigning oxidation numbers worksheet 1
- Oxidation number Exercise answer Key