Neutralization Reactions Worksheet

Understanding Neutralization Reactions
Neutralization reactions are a type of chemical reaction where an acid and a base react to form a salt and water. This reaction is often represented by the general equation:
Acid + Base → Salt + Water
Neutralization reactions are commonly seen in everyday life, such as when we take antacids to neutralize stomach acid or when we use baking soda to neutralize acidic spills.
Types of Neutralization Reactions
There are several types of neutralization reactions, including:
- Strong Acid-Strong Base Reactions: These reactions involve a strong acid and a strong base, resulting in a neutral solution.
- Weak Acid-Weak Base Reactions: These reactions involve a weak acid and a weak base, resulting in a solution that is slightly acidic or basic.
- Strong Acid-Weak Base Reactions: These reactions involve a strong acid and a weak base, resulting in a solution that is acidic.
- Weak Acid-Strong Base Reactions: These reactions involve a weak acid and a strong base, resulting in a solution that is basic.
Examples of Neutralization Reactions
Here are some examples of neutralization reactions:
- Hydrochloric Acid (HCl) and Sodium Hydroxide (NaOH):
HCl (acid) + NaOH (base) → NaCl (salt) + H2O (water)
- Sulfuric Acid (H2SO4) and Calcium Hydroxide (Ca(OH)2):
H2SO4 (acid) + Ca(OH)2 (base) → CaSO4 (salt) + 2H2O (water)
- Acetic Acid (CH3COOH) and Sodium Bicarbonate (NaHCO3):
CH3COOH (acid) + NaHCO3 (base) → CH3COONa (salt) + H2O (water) + CO2 (carbon dioxide)
Factors Affecting Neutralization Reactions
Several factors can affect the rate and extent of neutralization reactions, including:
- Concentration of Reactants: Increasing the concentration of reactants can increase the rate of reaction.
- Temperature: Increasing the temperature can increase the rate of reaction.
- Catalysts: Some substances can act as catalysts, speeding up the reaction without being consumed.
- Surface Area: Increasing the surface area of reactants can increase the rate of reaction.
Importance of Neutralization Reactions
Neutralization reactions have many practical applications, including:
- Antacids: Neutralizing stomach acid to relieve heartburn and indigestion.
- Wastewater Treatment: Neutralizing acidic or basic wastewater to make it safe for disposal.
- Food Industry: Neutralizing acidic or basic foods to make them safe for consumption.
- Pharmaceuticals: Neutralizing acidic or basic medications to make them safe for use.
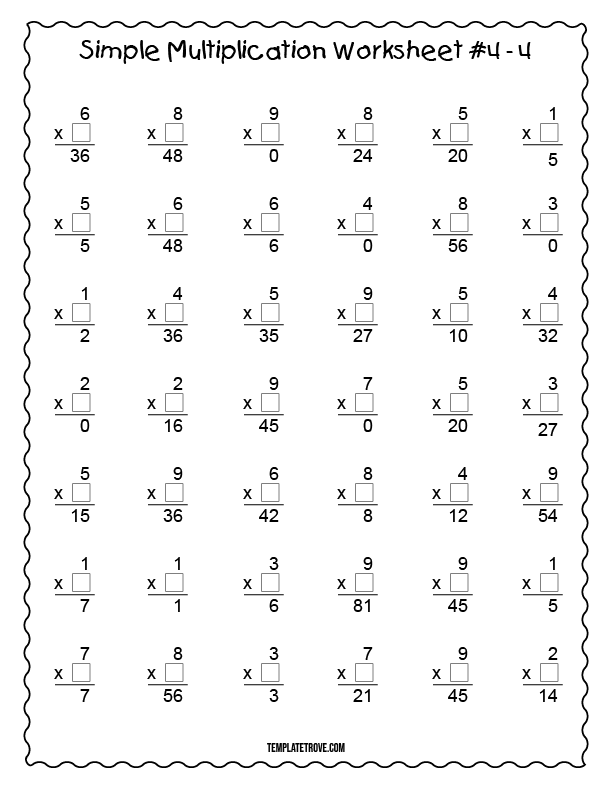
Worksheet
Complete the following worksheet to practice your understanding of neutralization reactions:

| Reaction | Acid | Base | Salt | Water |
|---|---|---|---|---|
| 1 | HCl | NaOH | ||
| 2 | H2SO4 | Ca(OH)2 | ||
| 3 | CH3COOH | NaHCO3 | ||
| 4 | HNO3 | KOH | ||
| 5 | H3PO4 | NaOH |
📝 Note: Fill in the blanks with the correct products of each reaction.
Conclusion
Neutralization reactions are an important type of chemical reaction that involves the reaction of an acid and a base to form a salt and water. Understanding these reactions is crucial in various fields, including chemistry, biology, and medicine. By completing the worksheet above, you can practice your understanding of neutralization reactions and apply your knowledge to real-world scenarios.
What is a neutralization reaction?
+A neutralization reaction is a type of chemical reaction where an acid and a base react to form a salt and water.
What are the types of neutralization reactions?
+There are several types of neutralization reactions, including strong acid-strong base reactions, weak acid-weak base reactions, strong acid-weak base reactions, and weak acid-strong base reactions.
What are some examples of neutralization reactions?
+Examples of neutralization reactions include the reaction of hydrochloric acid and sodium hydroxide, sulfuric acid and calcium hydroxide, and acetic acid and sodium bicarbonate.