6 Ways to Master Naming Ionic Compounds
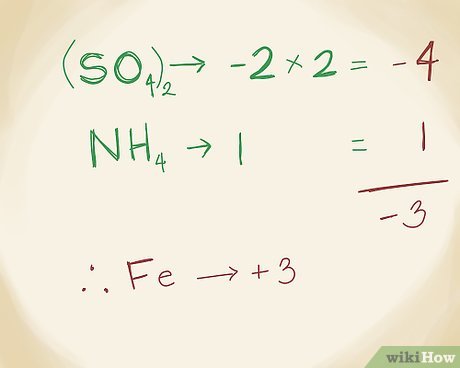
Naming compounds is an essential skill in chemistry, and mastering the art of naming ionic compounds is crucial for any student or professional in the field. Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. In this article, we will explore the six ways to master naming ionic compounds, including understanding the basics, learning the rules, and practicing with examples.
Understanding the Basics of Ionic Compounds
Before we dive into the six ways to master naming ionic compounds, it’s essential to understand the basics of ionic compounds. Ionic compounds are formed when a metal atom loses one or more electrons to form a positively charged ion (cation), while a nonmetal atom gains one or more electrons to form a negatively charged ion (anion). The electrostatic attraction between the oppositely charged ions holds them together, forming a strong chemical bond.
1. Learn the Rules for Naming Ionic Compounds
The first step to mastering the art of naming ionic compounds is to learn the rules. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming compounds, including ionic compounds. Here are the basic rules:
- Cations: The name of the cation is the same as the name of the element, with the exception of mercury, which is called mercuric (Hg2+) or mercurous (Hg2+).
- Anions: The name of the anion is the root of the element name, with the suffix “-ide” added. For example, the anion of oxygen is called oxide (O2-).
- Combining cations and anions: The name of the compound is formed by combining the name of the cation and the name of the anion. The name of the cation comes first, followed by the name of the anion.
For example, the compound formed by combining sodium (Na+) and chloride (Cl-) is called sodium chloride (NaCl).
2. Practice with Examples
Practice is key to mastering the art of naming ionic compounds. Here are some examples to get you started:
- Sodium nitrate (NaNO3): The cation is sodium (Na+), and the anion is nitrate (NO3-).
- Calcium carbonate (CaCO3): The cation is calcium (Ca2+), and the anion is carbonate (CO32-).
- Aluminum sulfate (Al2(SO4)3): The cation is aluminum (Al3+), and the anion is sulfate (SO42-).
3. Learn the Exceptions to the Rules
While the rules for naming ionic compounds are straightforward, there are some exceptions to the rules. Here are a few examples:
- Mercury: As mentioned earlier, mercury is called mercuric (Hg2+) or mercurous (Hg2+).
- Peroxides: Peroxides are compounds that contain the anion O22-. The name of the compound is formed by combining the name of the cation with the suffix “-peroxide”.
- Superoxides: Superoxides are compounds that contain the anion O2-. The name of the compound is formed by combining the name of the cation with the suffix “-superoxide”.
4. Use Prefixes to Indicate the Number of Ions
In some cases, the number of ions in an ionic compound may vary. To indicate the number of ions, prefixes are used. Here are the common prefixes:
- Mono-: one ion
- Di-: two ions
- Tri-: three ions
- Tetra-: four ions
- Penta-: five ions
- Hexa-: six ions
For example, the compound iron(III) oxide (Fe2O3) contains three iron ions and three oxygen ions.
5. Use Roman Numerals to Indicate the Charge of the Cation
In some cases, the charge of the cation may vary. To indicate the charge of the cation, Roman numerals are used. Here are the common Roman numerals:
- I: +1 charge
- II: +2 charge
- III: +3 charge
- IV: +4 charge
- V: +5 charge
- VI: +6 charge
For example, the compound iron(III) oxide (Fe2O3) contains iron ions with a +3 charge.
6. Practice with Polyatomic Ions
Polyatomic ions are ions that contain more than one element. Here are a few examples of polyatomic ions:
- Ammonium (NH4+): a polyatomic ion containing nitrogen and hydrogen
- Sulfate (SO42-): a polyatomic ion containing sulfur and oxygen
- Phosphate (PO43-): a polyatomic ion containing phosphorus and oxygen
To name compounds that contain polyatomic ions, the same rules apply. For example, the compound ammonium sulfate (NH4)2SO4 contains the polyatomic ion ammonium (NH4+) and the polyatomic ion sulfate (SO42-).
📝 Note: Polyatomic ions are often used in combination with other ions to form compounds.
By following these six steps, you can master the art of naming ionic compounds. Remember to practice with examples, learn the exceptions to the rules, and use prefixes and Roman numerals to indicate the number of ions and the charge of the cation.
What is the rule for naming cations?
+The name of the cation is the same as the name of the element, with the exception of mercury, which is called mercuric (Hg2+) or mercurous (Hg2+).
What is the rule for naming anions?
+The name of the anion is the root of the element name, with the suffix “-ide” added. For example, the anion of oxygen is called oxide (O2-).
How do I indicate the number of ions in an ionic compound?
+To indicate the number of ions, prefixes are used. For example, the prefix “di-” indicates two ions, while the prefix “tri-” indicates three ions.