Ionic Compound Names and Formulas Made Easy
Understanding Ionic Compounds
Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. The electrostatic attraction between these oppositely charged ions holds them together, creating a strong chemical bond. Ionic compounds are typically composed of a metal cation and a nonmetal anion.
The Basics of Ionic Compound Nomenclature
Ionic compound names are derived from the names of the ions that make up the compound. The name of the cation comes first, followed by the name of the anion. Cations are typically named using the element’s name, while anions are named by changing the ending of the element’s name to -ide.
💡 Note: Some cations have variable charges, which must be indicated in the compound name using a Roman numeral in parentheses.
Monatomic Ions
Monatomic ions are ions that consist of a single atom. These ions can be either cations or anions.
Common Monatomic Cations:
- Sodium (Na+)
- Calcium (Ca2+)
- Aluminum (Al3+)
Common Monatomic Anions:
- Chloride (Cl-)
- Oxide (O2-)
- Nitride (N3-)
Polyatomic Ions
Polyatomic ions are ions that consist of multiple atoms. These ions can also be either cations or anions.
Common Polyatomic Ions:
- Ammonium (NH4+)
- Hydroxide (OH-)
- Carbonate (CO32-)
Naming Ionic Compounds
To name an ionic compound, follow these steps:
- Write the name of the cation. If the cation has a variable charge, indicate the charge using a Roman numeral in parentheses.
- Write the name of the anion. Change the ending of the element’s name to -ide.
- Combine the names of the cation and anion.
Examples:
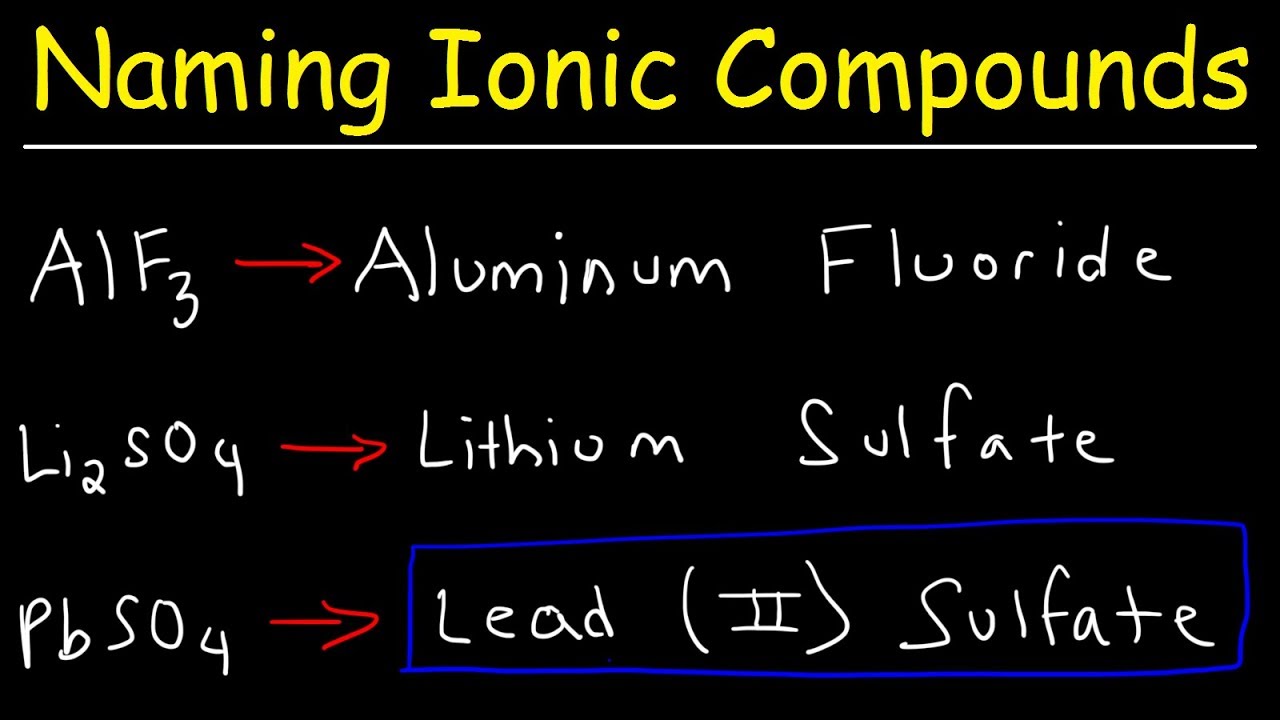
- Sodium chloride (NaCl) - composed of a sodium cation and a chloride anion
- Calcium carbonate (CaCO3) - composed of a calcium cation and a carbonate anion
- Aluminum oxide (Al2O3) - composed of an aluminum cation and an oxide anion
Writing Ionic Compound Formulas
To write the formula of an ionic compound, follow these steps:
- Write the symbol of the cation. If the cation has a variable charge, indicate the charge using a Roman numeral in parentheses.
- Write the symbol of the anion.
- Balance the charges. The total positive charge of the cation(s) must equal the total negative charge of the anion(s).
Examples:

| Compound | Formula |
|---|---|
| Sodium chloride | NaCl |
| Calcium carbonate | CaCO3 |
| Aluminum oxide | Al2O3 |
Conclusion
Naming and writing formulas for ionic compounds can seem intimidating at first, but by understanding the basics of ionic compound nomenclature and following a few simple steps, you can easily master this skill. Remember to always indicate the charge of the cation using a Roman numeral in parentheses, and to balance the charges when writing the formula.
What is the difference between a monatomic ion and a polyatomic ion?
+A monatomic ion is an ion that consists of a single atom, while a polyatomic ion is an ion that consists of multiple atoms.
How do I indicate the charge of a cation in the compound name?
+The charge of a cation is indicated using a Roman numeral in parentheses, such as (I), (II), or (III).
What is the rule for naming anions?
+The rule for naming anions is to change the ending of the element’s name to -ide.