Mole Ratio Worksheet: Balancing Chemical Equations Made Easy

Understanding Mole Ratio and Its Importance in Balancing Chemical Equations
Balancing chemical equations is a fundamental concept in chemistry, and mole ratio plays a crucial role in achieving this balance. In this article, we will delve into the world of mole ratio, explore its significance, and provide a comprehensive worksheet to help you master the art of balancing chemical equations.
What is Mole Ratio?
Mole ratio is a mathematical representation of the number of moles of each reactant and product in a chemical reaction. It is a ratio of the coefficients of the balanced chemical equation. To calculate the mole ratio, you need to know the number of moles of each substance involved in the reaction.
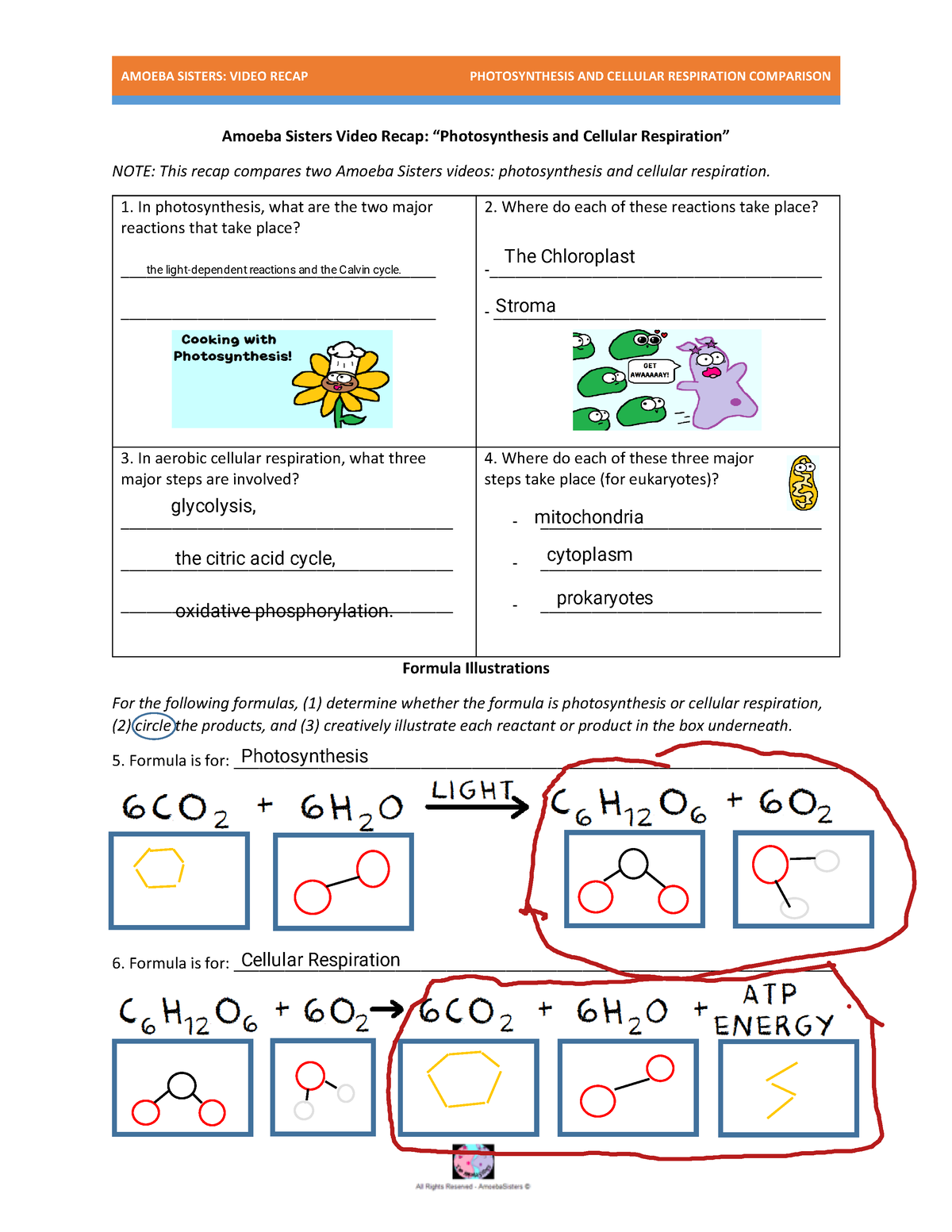
Why is Mole Ratio Important?
Mole ratio is essential in balancing chemical equations because it helps you:
- Determine the correct coefficients: By calculating the mole ratio, you can determine the correct coefficients for each reactant and product, ensuring that the equation is balanced.
- Predict the amount of reactants and products: Mole ratio allows you to predict the amount of reactants required and the amount of products formed in a reaction.
- Understand the stoichiometry of the reaction: Mole ratio provides insight into the stoichiometry of the reaction, helping you understand the quantitative relationship between the reactants and products.
How to Calculate Mole Ratio
Calculating mole ratio is a straightforward process. Here’s a step-by-step guide:
- Write the unbalanced equation: Start by writing the unbalanced chemical equation.
- Count the atoms: Count the number of atoms of each element on both the reactant and product sides.
- Determine the coefficients: Determine the coefficients needed to balance the equation.
- Calculate the mole ratio: Calculate the mole ratio by dividing the coefficient of each substance by the smallest coefficient.
📝 Note: The smallest coefficient is usually 1, but it can be a different number if the equation requires it.
Mole Ratio Worksheet
Here’s a comprehensive worksheet to help you practice calculating mole ratios:
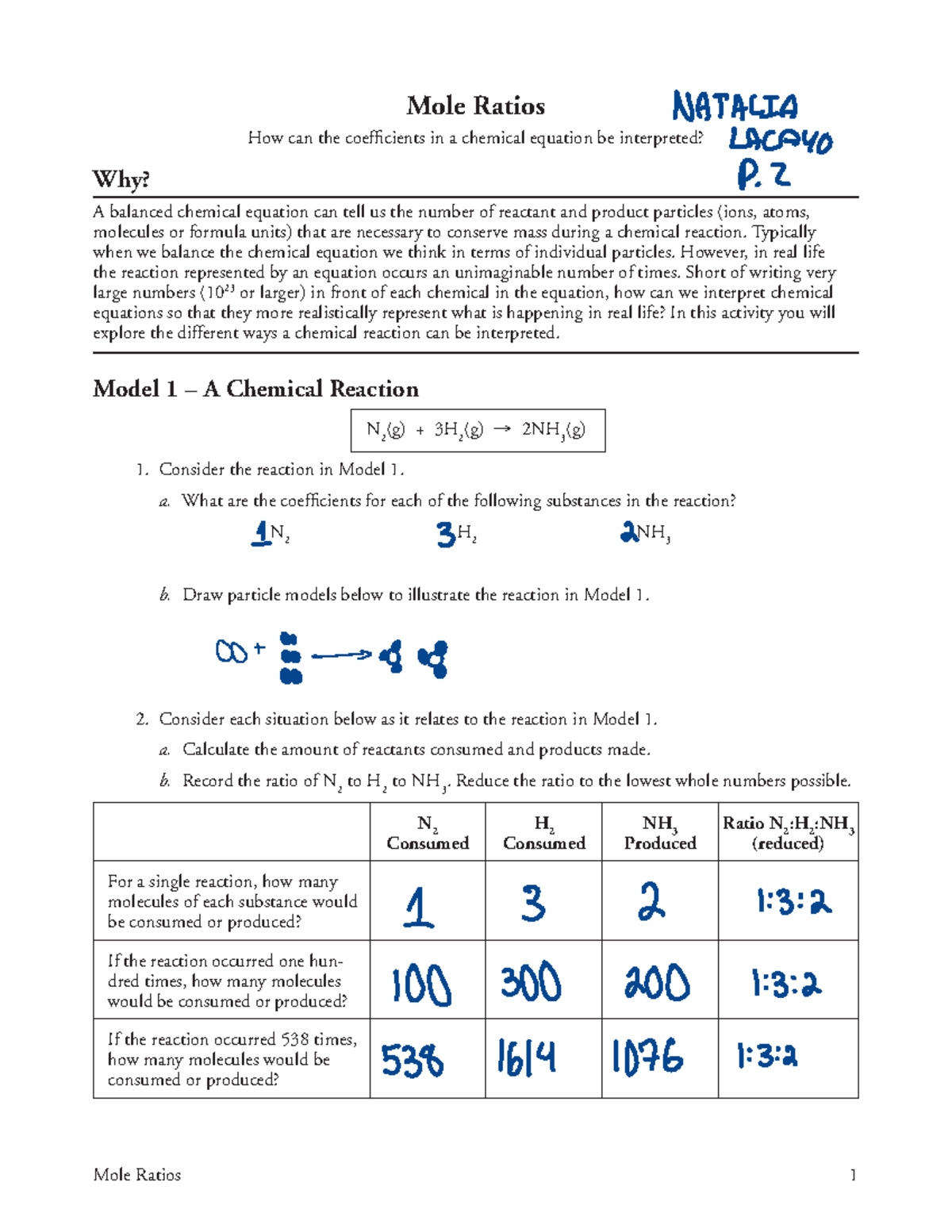
| Reaction | Mole Ratio |
|---|---|
| 2Na + Cl2 → 2NaCl | 1:1:2 |
| Ca + HCl → CaCl2 + H2 | 1:2:1:1 |
| 2Al + Fe2O3 → 2Fe + Al2O3 | 2:1:2:1 |
| CH4 + 2O2 → CO2 + 2H2O | 1:2:1:2 |
| 2K + Cl2 → 2KCl | 1:1:2 |
Instructions:
- Write the balanced chemical equation for each reaction.
- Calculate the mole ratio for each reaction.
- Verify your answers using the mole ratio provided.
Examples and Solutions
Let’s solve the first reaction:
2Na + Cl2 → 2NaCl
Step 1: Write the unbalanced equation.
2Na + Cl2 → 2NaCl
Step 2: Count the atoms.
| Element | Reactants | Products |
|---|---|---|
| Na | 2 | 2 |
| Cl | 2 | 2 |
Step 3: Determine the coefficients.
The equation is already balanced, so the coefficients are 2 for Na and 1 for Cl2.
Step 4: Calculate the mole ratio.
Mole ratio = 2:1:2
Answer: The mole ratio is 2:1:2.
Tips and Tricks
- Use the smallest coefficient: When calculating the mole ratio, use the smallest coefficient as the divisor.
- Simplify the ratio: Simplify the mole ratio by dividing each number by the greatest common divisor.
- Verify your answers: Verify your answers by plugging the mole ratio back into the balanced equation.
Common Mistakes to Avoid
- Incorrect coefficients: Double-check your coefficients to ensure they are correct.
- Incorrect counting: Make sure to count the atoms correctly, including any subscripts.
- Not simplifying the ratio: Simplify the mole ratio to ensure it is in the simplest form.
By following these tips and practicing with the worksheet, you’ll become a pro at calculating mole ratios and balancing chemical equations in no time!
What is the purpose of calculating mole ratio?
+Calculating mole ratio helps determine the correct coefficients for each reactant and product, predicts the amount of reactants and products, and provides insight into the stoichiometry of the reaction.
How do I calculate mole ratio?
+Calculate mole ratio by dividing the coefficient of each substance by the smallest coefficient.
What are common mistakes to avoid when calculating mole ratio?
+Common mistakes include incorrect coefficients, incorrect counting, and not simplifying the ratio.
In conclusion, mastering mole ratio is essential for balancing chemical equations. By understanding the concept of mole ratio and practicing with the worksheet, you’ll become proficient in calculating mole ratios and balancing chemical equations. Remember to use the smallest coefficient, simplify the ratio, and verify your answers to ensure accuracy. Happy practicing!