Mole Mole Stoichiometry Made Easy
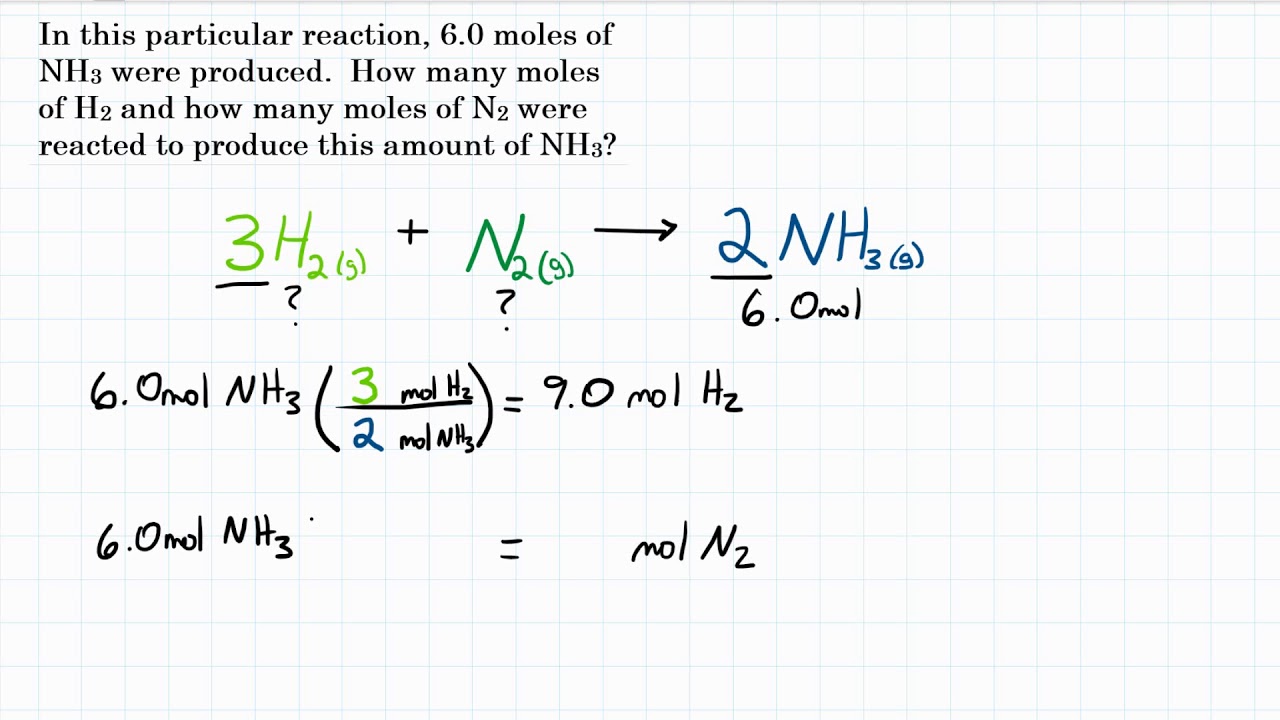
Introduction to Mole Mole Stoichiometry
Mole mole stoichiometry, also known as mole-to-mole stoichiometry, is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It is based on the mole concept, which is a way of expressing the amount of a substance. In this article, we will explore the basics of mole mole stoichiometry and provide a step-by-step guide on how to apply it in solving chemical reaction problems.
Understanding the Mole Concept
Before we dive into mole mole stoichiometry, it is essential to understand the mole concept. A mole is a unit of measurement that represents 6.022 x 10^23 particles, such as atoms or molecules. The mole concept allows us to express the amount of a substance in a way that is independent of its physical properties.
The Mole Mole Ratio
The mole mole ratio is a fundamental concept in mole mole stoichiometry. It is the ratio of the number of moles of one substance to the number of moles of another substance in a balanced chemical equation. The mole mole ratio is used to predict the amount of reactants required to produce a certain amount of product.
Calculating Mole Mole Ratios
To calculate the mole mole ratio, we need to follow these steps:
- Write the balanced chemical equation for the reaction.
- Identify the reactants and products.
- Calculate the number of moles of each reactant and product using the mole concept.
- Divide the number of moles of one substance by the number of moles of another substance to get the mole mole ratio.
📝 Note: Make sure to use the coefficients in the balanced chemical equation to calculate the mole mole ratio.
Example Problem 1
Consider the following balanced chemical equation:
2H2 + O2 → 2H2O
Calculate the mole mole ratio of H2 to O2.
- Write the balanced chemical equation.
- Identify the reactants and products: H2 (reactant), O2 (reactant), H2O (product).
- Calculate the number of moles of each reactant and product: 2 mol H2, 1 mol O2, 2 mol H2O.
- Divide the number of moles of H2 by the number of moles of O2: 2 mol H2 / 1 mol O2 = 2.
Therefore, the mole mole ratio of H2 to O2 is 2:1.
Using Mole Mole Ratios to Solve Problems
Mole mole ratios can be used to solve a variety of problems in chemistry, including:
- Calculating the amount of reactant required to produce a certain amount of product.
- Calculating the amount of product produced from a certain amount of reactant.
- Determining the limiting reactant in a reaction.
Example Problem 2
Consider the following problem:
How many moles of O2 are required to produce 4 mol of H2O in the reaction 2H2 + O2 → 2H2O?
- Use the mole mole ratio to solve the problem: 2 mol H2 / 1 mol O2 = 4 mol H2O / x mol O2.
- Solve for x: x = 2 mol O2.
Therefore, 2 mol of O2 are required to produce 4 mol of H2O.
Summary of Key Points
In summary, mole mole stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. The mole mole ratio is a key concept in mole mole stoichiometry, and it is used to predict the amount of reactants required to produce a certain amount of product. By following the steps outlined in this article, you can apply mole mole stoichiometry to solve a variety of problems in chemistry.
What is the definition of mole mole stoichiometry?
+Mole mole stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction.
How do you calculate the mole mole ratio?
+To calculate the mole mole ratio, divide the number of moles of one substance by the number of moles of another substance in a balanced chemical equation.
What is the mole concept?
+The mole concept is a way of expressing the amount of a substance, and it represents 6.022 x 10^23 particles, such as atoms or molecules.