Mole Conversions Made Easy Worksheet
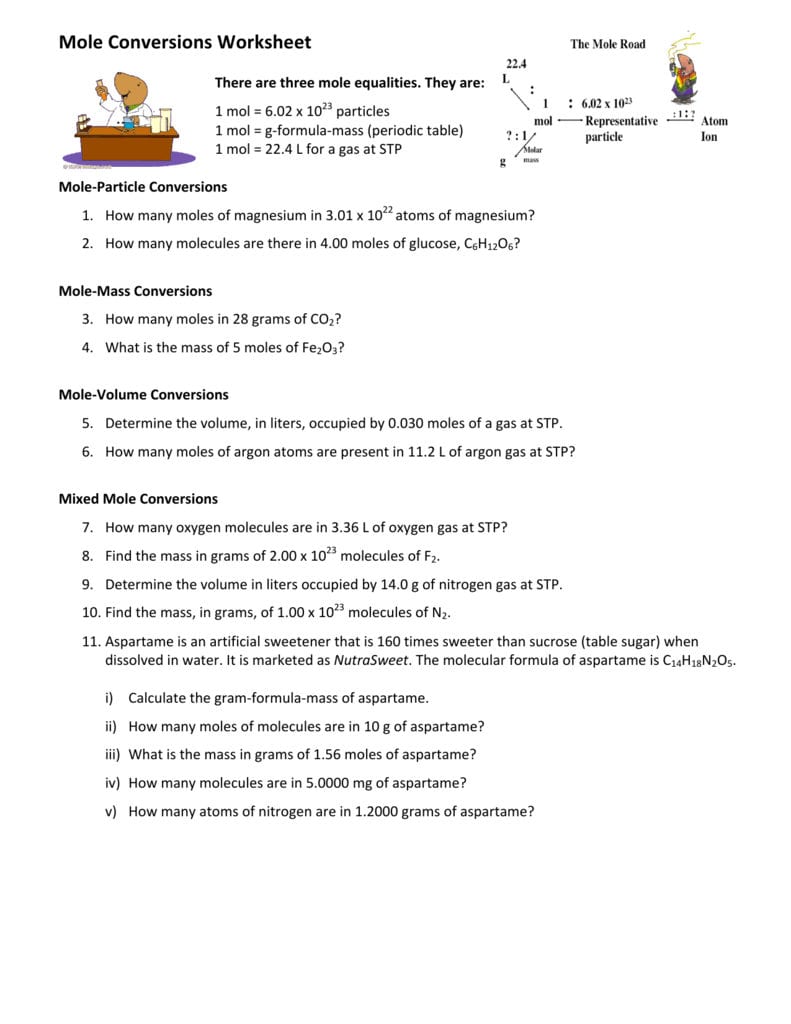
Mastering Mole Conversions: A Step-by-Step Guide
Mole conversions are a fundamental concept in chemistry, and mastering them is crucial for success in the subject. However, many students struggle with these conversions, finding them confusing and daunting. In this guide, we’ll break down the process of mole conversions into simple, easy-to-follow steps. By the end of this tutorial, you’ll be able to perform mole conversions with confidence and accuracy.
Understanding the Mole Concept
Before we dive into the conversions, let’s quickly review the concept of a mole. A mole is a unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules). This number is known as Avogadro’s number. The mole is used to express the amount of a substance in a way that’s independent of its physical properties, such as mass or volume.
Mole Conversion Formulas
To perform mole conversions, you’ll need to use the following formulas:
- moles = mass / molar mass
- moles = volume / molar volume
- moles = number of particles / Avogadro’s number
These formulas will be used throughout the conversion process.
Step-by-Step Mole Conversions
Now that we have the formulas, let’s go through the step-by-step process of mole conversions.
Step 1: Identify the Given Information
Start by identifying the given information, which can be in the form of mass, volume, or number of particles. Make sure you understand the units of the given information.
Step 2: Determine the Conversion Type
Determine the type of conversion you need to perform. Is it a mass-to-mole conversion, a volume-to-mole conversion, or a particle-to-mole conversion? This will help you choose the correct formula.
Step 3: Plug in the Values
Plug in the given values into the chosen formula. Make sure you’re using the correct units and that you’re canceling out the correct units.
Step 4: Calculate the Answer
Calculate the answer using the formula. Make sure you’re using the correct number of significant figures.
Step 5: Check Your Answer
Check your answer to ensure it makes sense. If the answer seems too large or too small, re-check your calculations.
Example Problems
Let’s go through some example problems to illustrate the mole conversion process.
Problem 1: A sample of oxygen gas has a mass of 32.0 g. How many moles of oxygen are present?
- Given information: mass = 32.0 g
- Conversion type: mass-to-mole conversion
- Formula: moles = mass / molar mass
- Molar mass of oxygen: 32.0 g/mol
- Calculation: moles = 32.0 g / 32.0 g/mol = 1.00 mol
Problem 2: A sample of helium gas has a volume of 25.0 L at standard temperature and pressure (STP). How many moles of helium are present?
- Given information: volume = 25.0 L
- Conversion type: volume-to-mole conversion
- Formula: moles = volume / molar volume
- Molar volume at STP: 22.4 L/mol
- Calculation: moles = 25.0 L / 22.4 L/mol = 1.12 mol
Problem 3: A sample of carbon dioxide contains 6.02 x 10^23 molecules. How many moles of carbon dioxide are present?
- Given information: number of particles = 6.02 x 10^23 molecules
- Conversion type: particle-to-mole conversion
- Formula: moles = number of particles / Avogadro’s number
- Calculation: moles = 6.02 x 10^23 molecules / 6.022 x 10^23 particles/mol = 1.00 mol
Common Mistakes to Avoid
When performing mole conversions, there are several common mistakes to avoid:
- Incorrect units: Make sure you’re using the correct units for the given information and the conversion formula.
- Incorrect significant figures: Make sure you’re using the correct number of significant figures in your calculations.
- Rounding errors: Avoid rounding errors by using the correct number of decimal places in your calculations.
💡 Note: Always double-check your calculations to ensure accuracy and avoid errors.
Practice Makes Perfect
Mole conversions can seem daunting at first, but with practice, you’ll become more confident and proficient. Try practicing with different types of problems and conversions to reinforce your understanding.
Additional Tips
- Use conversion factors: Conversion factors can help you simplify complex conversions and avoid errors.
- Check your units: Always check your units to ensure you’re using the correct ones.
- Practice, practice, practice: The more you practice, the more comfortable you’ll become with mole conversions.
What is the definition of a mole?
+A mole is a unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules).
What is the formula for mass-to-mole conversion?
+moles = mass / molar mass
What is the most common mistake to avoid in mole conversions?
+Incorrect units are the most common mistake to avoid in mole conversions.
In conclusion, mole conversions are a fundamental concept in chemistry that can seem daunting at first, but with practice and understanding, you’ll become more confident and proficient. By following the step-by-step guide outlined in this tutorial, you’ll be able to perform mole conversions with accuracy and ease. Remember to practice regularly and avoid common mistakes to become a master of mole conversions.
Related Terms:
- Mole Conversions Worksheet with answers
- mass-mole conversions worksheet answer key
- Mole conversion Worksheet pdf
- Mixed Mole Conversions Worksheet
- Multi step mole Conversions Worksheet