Mole Conversion Worksheet
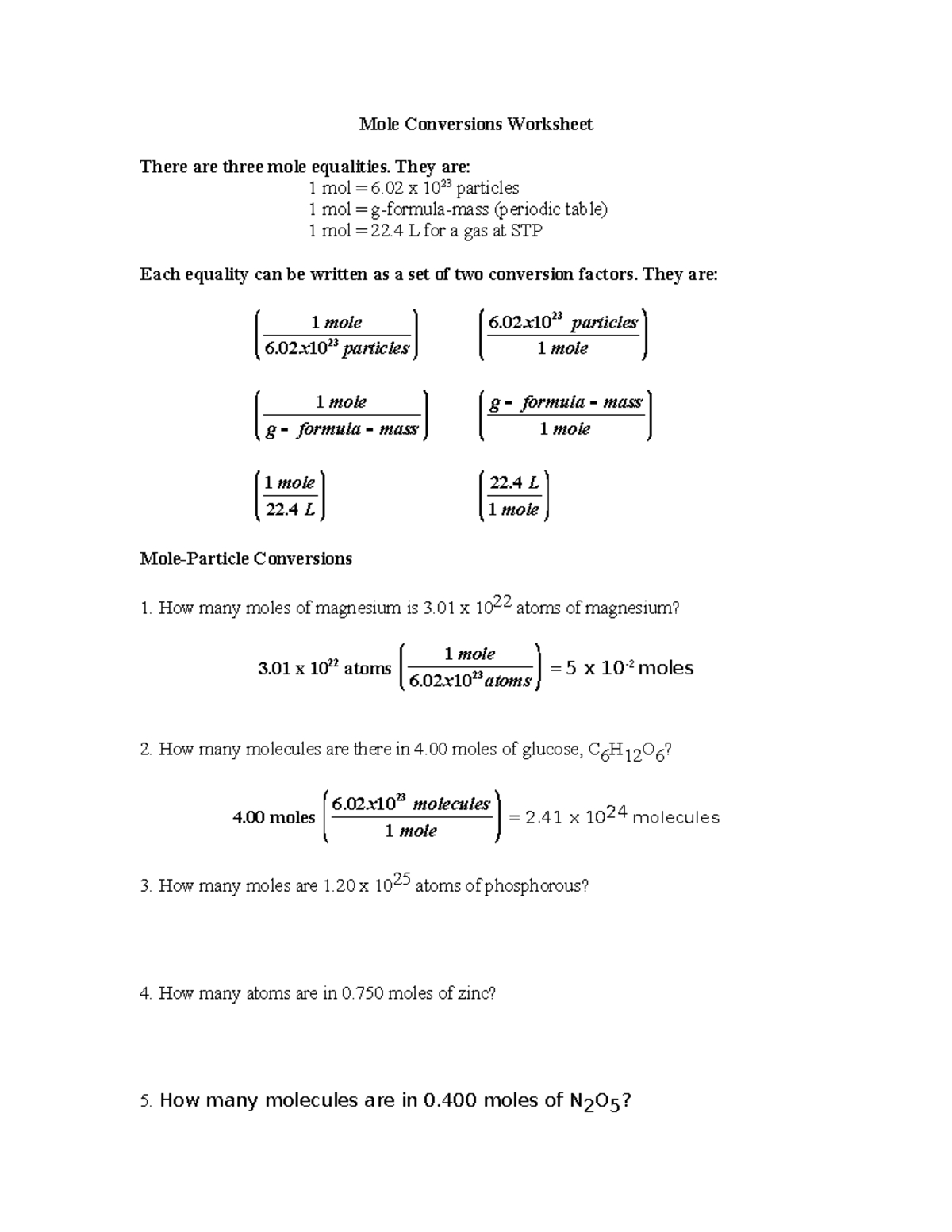
Understanding Mole Conversion: A Comprehensive Guide
Mole conversion is a fundamental concept in chemistry that involves converting between different units of measurement, such as grams to moles or moles to particles. This process is crucial in various chemical calculations, including stoichiometry, chemical reactions, and thermodynamics. In this worksheet, we will delve into the world of mole conversion, exploring the concepts, formulas, and techniques involved.
Mole Conversion Basics
A mole (mol) is the unit of measurement in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules. The mole is used to express the amount of a substance, and it is essential to understand how to convert between different units.
Key Concepts:
- Molar mass: The mass of one mole of a substance, typically expressed in grams per mole (g/mol).
- Mole ratio: The ratio of moles of one substance to another in a chemical reaction.
- Conversion factor: A ratio used to convert between different units, such as grams to moles.
Converting Grams to Moles
To convert grams to moles, you need to know the molar mass of the substance. The formula for this conversion is:
moles = mass (g) / molar mass (g/mol)
For example, if you have 25.0 grams of sodium chloride (NaCl) and the molar mass is 58.44 g/mol, how many moles do you have?
moles = 25.0 g / 58.44 g/mol = 0.428 mol
Table: Molar Masses of Common Substances
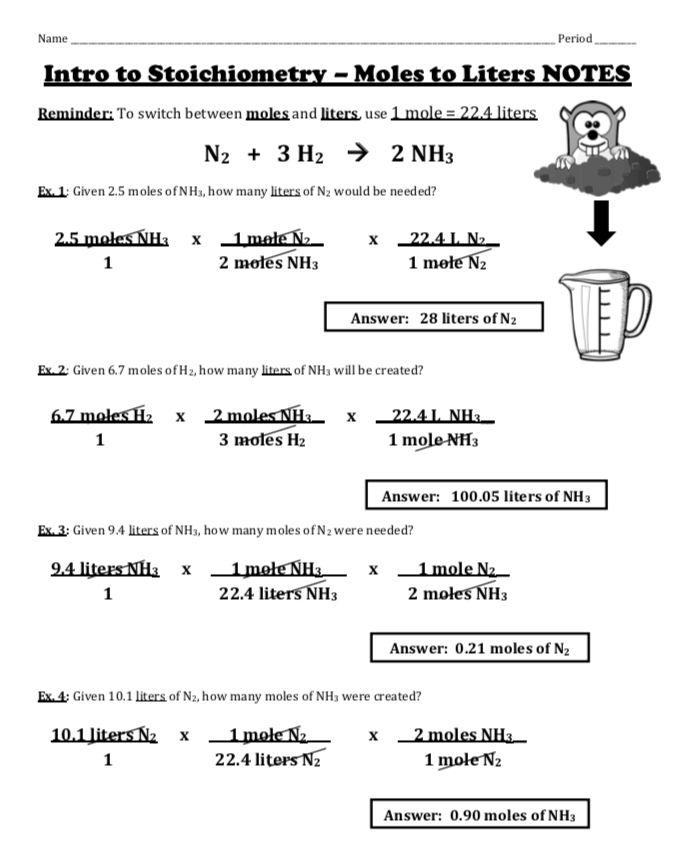
| Substance | Molar Mass (g/mol) |
|---|---|
| Sodium chloride (NaCl) | 58.44 |
| Water (H2O) | 18.02 |
| Carbon dioxide (CO2) | 44.01 |
| Oxygen (O2) | 32.00 |
📝 Note: Make sure to use the correct molar mass for the substance you are working with.
Converting Moles to Particles
To convert moles to particles, you can use the Avogadro’s number (6.022 x 10^23 particles/mol).
particles = moles x Avogadro’s number
For example, if you have 0.428 mol of sodium chloride (NaCl), how many particles do you have?
particles = 0.428 mol x 6.022 x 10^23 particles/mol = 2.58 x 10^23 particles
Converting Between Different Units
When working with chemical reactions, you may need to convert between different units, such as liters to grams or grams to milliliters.
- Liters to grams: Use the density of the substance to convert between liters and grams. density = mass / volume
- Grams to milliliters: Use the conversion factor 1 g = 1 mL (approximately) for water-like substances.
Example: Converting Liters to Grams
If you have 2.5 liters of water and the density is 1.00 g/mL, how many grams do you have?
mass = density x volume = 1.00 g/mL x 2500 mL = 2500 g
Practice Problems
Try the following practice problems to reinforce your understanding of mole conversion:
- Convert 50.0 grams of carbon dioxide (CO2) to moles.
- Convert 0.250 mol of oxygen (O2) to particles.
- Convert 1.50 liters of water to grams.
Answers
- 50.0 grams of carbon dioxide (CO2) = 1.14 mol
- 0.250 mol of oxygen (O2) = 1.51 x 10^23 particles
- 1.50 liters of water = 1500 g
What is the definition of a mole?
+A mole is the unit of measurement in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules.
How do I convert grams to moles?
+Use the formula: moles = mass (g) / molar mass (g/mol)
What is Avogadro's number used for?
+Avogadro's number is used to convert moles to particles: particles = moles x Avogadro's number
In conclusion, mole conversion is a critical concept in chemistry that requires a thorough understanding of the formulas, techniques, and units involved. By mastering these concepts, you will be well-equipped to tackle a wide range of chemical problems and calculations.