5 Easy Steps to Master Molarity Worksheet Answer Key

Mastering Molarity Worksheets
Molarity is a fundamental concept in chemistry that can be challenging for students to grasp. With practice and the right resources, however, it can become second nature. In this article, we will walk you through 5 easy steps to master molarity worksheets, complete with examples and a review of key concepts.
Step 1: Understand the Definition of Molarity
Molarity is defined as the number of moles of a substance per liter of solution. It is expressed in units of moles per liter (mol/L) and is often denoted by the symbol “M”. Molarity is a measure of the concentration of a solution and is commonly used in chemistry and biology.
Step 2: Learn the Molarity Formula
The molarity formula is:
M = moles of solute / liters of solution
Where:
- M is the molarity of the solution
- moles of solute is the number of moles of the substance being dissolved
- liters of solution is the total volume of the solution in liters
Step 3: Practice Calculating Molarity
Now that you understand the definition and formula of molarity, it’s time to practice calculating it. Here’s an example:
Problem: What is the molarity of a solution that contains 2.5 moles of sodium chloride (NaCl) in 0.5 liters of water?
Solution:
M = moles of solute / liters of solution M = 2.5 mol / 0.5 L M = 5 mol/L
Answer: The molarity of the solution is 5 mol/L.
Step 4: Understand Dilution and Concentration
When working with molarity, it’s essential to understand dilution and concentration. Dilution occurs when a solution is mixed with a solvent to decrease its concentration, while concentration occurs when a solution is mixed with a solute to increase its concentration.
Here’s an example:
Problem: If you have a 2 mol/L solution of sodium hydroxide (NaOH) and you dilute it to 1 liter with water, what is the new molarity?
Solution:
Initial molarity = 2 mol/L Initial volume = 0.5 L Final volume = 1 L
To find the new molarity, you need to calculate the number of moles of NaOH in the final solution:
moles of NaOH = initial molarity x initial volume moles of NaOH = 2 mol/L x 0.5 L moles of NaOH = 1 mol
New molarity = moles of NaOH / final volume New molarity = 1 mol / 1 L New molarity = 1 mol/L
Answer: The new molarity of the solution is 1 mol/L.
Step 5: Review and Practice with Molarity Worksheets
The final step to mastering molarity worksheets is to review and practice with sample problems. You can find many online resources that offer molarity worksheets with answers. Practice regularly, and you’ll become proficient in calculating molarity in no time.
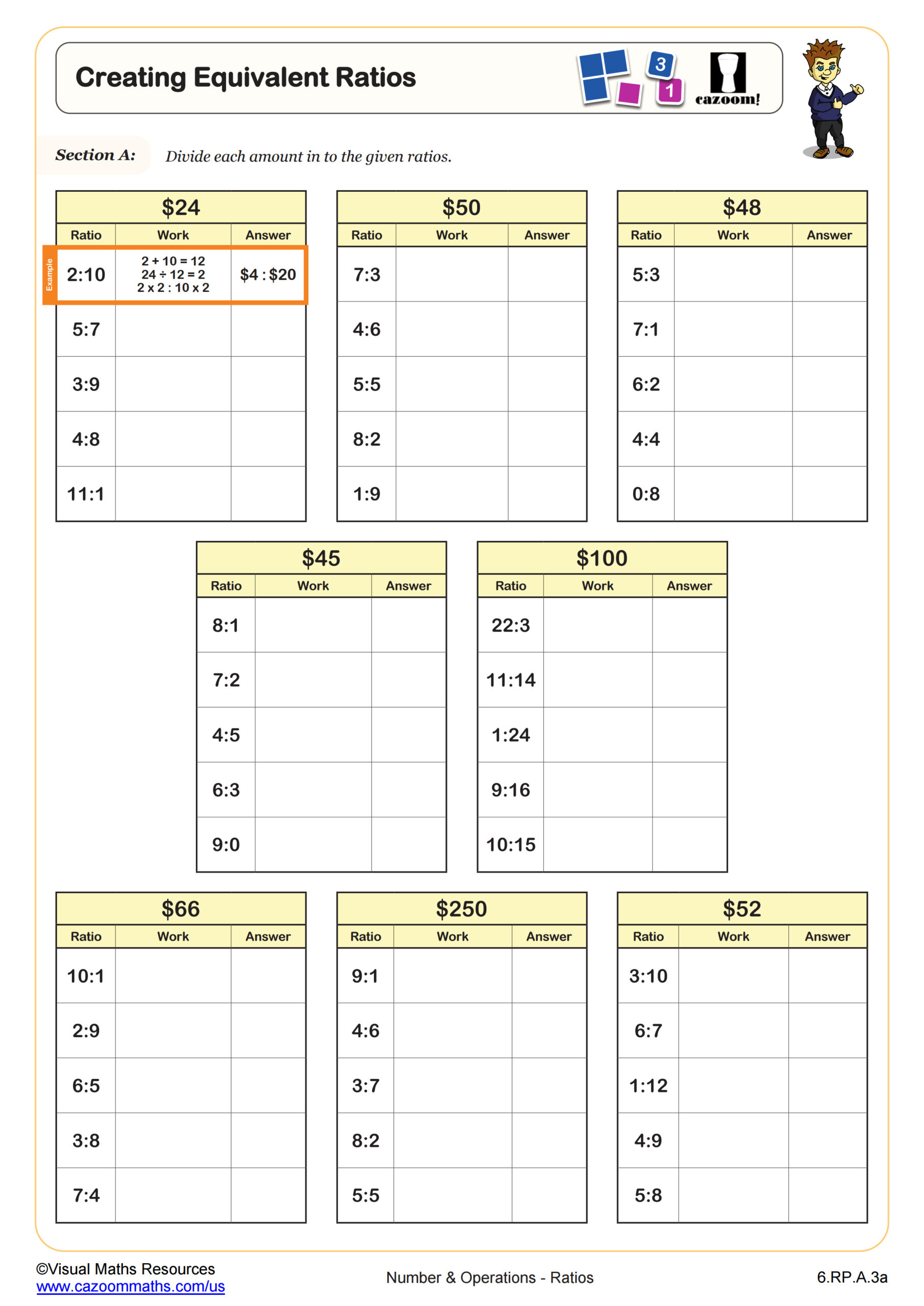
Molarity Worksheet Answer Key
Here’s a sample molarity worksheet with answers:

| Problem | Answer |
|---|---|
| What is the molarity of a solution that contains 3.5 moles of potassium nitrate (KNO3) in 2 liters of water? | 1.75 mol/L |
| If you have a 1.5 mol/L solution of hydrochloric acid (HCl) and you dilute it to 2 liters with water, what is the new molarity? | 0.75 mol/L |
| What is the molarity of a solution that contains 0.25 moles of calcium chloride (CaCl2) in 0.5 liters of water? | 0.5 mol/L |
Notes
- Make sure to always check the units of the given values and the desired answer.
- When diluting or concentrating a solution, always calculate the number of moles of the solute.
- Practice regularly to become proficient in calculating molarity.
What is the definition of molarity?
+Molarity is defined as the number of moles of a substance per liter of solution.
What is the molarity formula?
+M = moles of solute / liters of solution
What is dilution and concentration in the context of molarity?
+Dilution occurs when a solution is mixed with a solvent to decrease its concentration, while concentration occurs when a solution is mixed with a solute to increase its concentration.
By following these 5 easy steps, you’ll be well on your way to mastering molarity worksheets. Remember to practice regularly and review key concepts to become proficient in calculating molarity.