5 Easy Molarity Calculation Steps
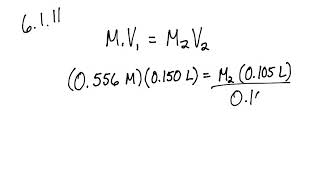
Understanding Molarity: A Step-by-Step Guide to Calculations
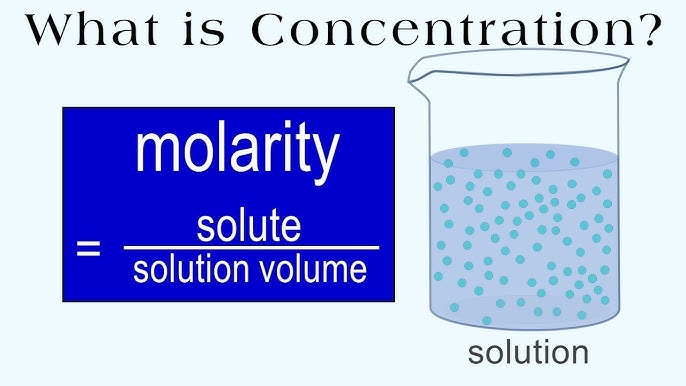
Molarity is a fundamental concept in chemistry that describes the concentration of a solution. It is defined as the number of moles of solute per liter of solution. Calculating molarity is a crucial skill for chemists, biologists, and anyone working with solutions. In this article, we will break down the molarity calculation process into 5 easy steps.
Step 1: Identify the Given Information
To calculate molarity, you need to know the following information:
- The number of moles of solute (n)
- The volume of the solution in liters (V)
- The molar mass of the solute (optional)
This information can be found in the problem statement or by measuring the solution and solute.
📝 Note: Make sure to check the units of the given information. The volume should be in liters (L), and the number of moles should be in moles (mol).
Step 2: Determine the Molar Mass of the Solute (if necessary)
If the molar mass of the solute is not given, you need to calculate it. The molar mass is the sum of the atomic masses of the elements in the compound. You can find the atomic masses on the periodic table.
For example, the molar mass of sodium chloride (NaCl) is:
- Sodium (Na): 22.99 g/mol
- Chlorine (Cl): 35.45 g/mol
- Molar mass of NaCl: 22.99 + 35.45 = 58.44 g/mol
Step 3: Calculate the Number of Moles of Solute
If the number of moles of solute is not given, you need to calculate it using the formula:
n = mass of solute / molar mass
For example, if you have 10 grams of sodium chloride and the molar mass is 58.44 g/mol, the number of moles is:
n = 10 g / 58.44 g/mol = 0.171 mol
Step 4: Calculate the Molarity
Now that you have the number of moles of solute and the volume of the solution, you can calculate the molarity using the formula:
M = n / V
For example, if you have 0.171 mol of sodium chloride in 0.5 liters of solution, the molarity is:
M = 0.171 mol / 0.5 L = 0.342 M
Step 5: Express the Answer in the Correct Units
Finally, make sure to express the answer in the correct units, which is moles per liter (M).
For example, the molarity of the solution is 0.342 M.
| Given Information | Calculated Values |
|---|---|
| Mass of solute: 10 g | Number of moles: 0.171 mol |
| Molar mass: 58.44 g/mol | Molarity: 0.342 M |
| Volume of solution: 0.5 L |
In conclusion, calculating molarity is a straightforward process that requires identifying the given information, determining the molar mass of the solute (if necessary), calculating the number of moles of solute, calculating the molarity, and expressing the answer in the correct units.
The key takeaways from this article are:
- Molarity is defined as the number of moles of solute per liter of solution.
- The formula for calculating molarity is M = n / V.
- Make sure to check the units of the given information and express the answer in the correct units.
What is the difference between molarity and molality?
+Molarity is defined as the number of moles of solute per liter of solution, while molality is defined as the number of moles of solute per kilogram of solvent.
How do I calculate the molar mass of a compound?
+The molar mass of a compound is the sum of the atomic masses of the elements in the compound. You can find the atomic masses on the periodic table.
What are the units of molarity?
+The units of molarity are moles per liter (M).
Related Terms:
- Amonium karbonat
- Kalium karbonat
- Kalium fluorida
- Natrium karbonat
- Molarity calculations worksheet with answers
- Molarity Worksheet With answers pdf