5 Tips for Mastering Limiting Reagent Stoichiometry
Understanding Limiting Reagent Stoichiometry
Limiting reagent stoichiometry is a fundamental concept in chemistry that can be daunting for many students. It requires a deep understanding of chemical reactions, mole ratios, and the limiting reagent concept. In this article, we will provide you with 5 tips to help you master limiting reagent stoichiometry.
Tip 1: Understand the Concept of Limiting Reagent
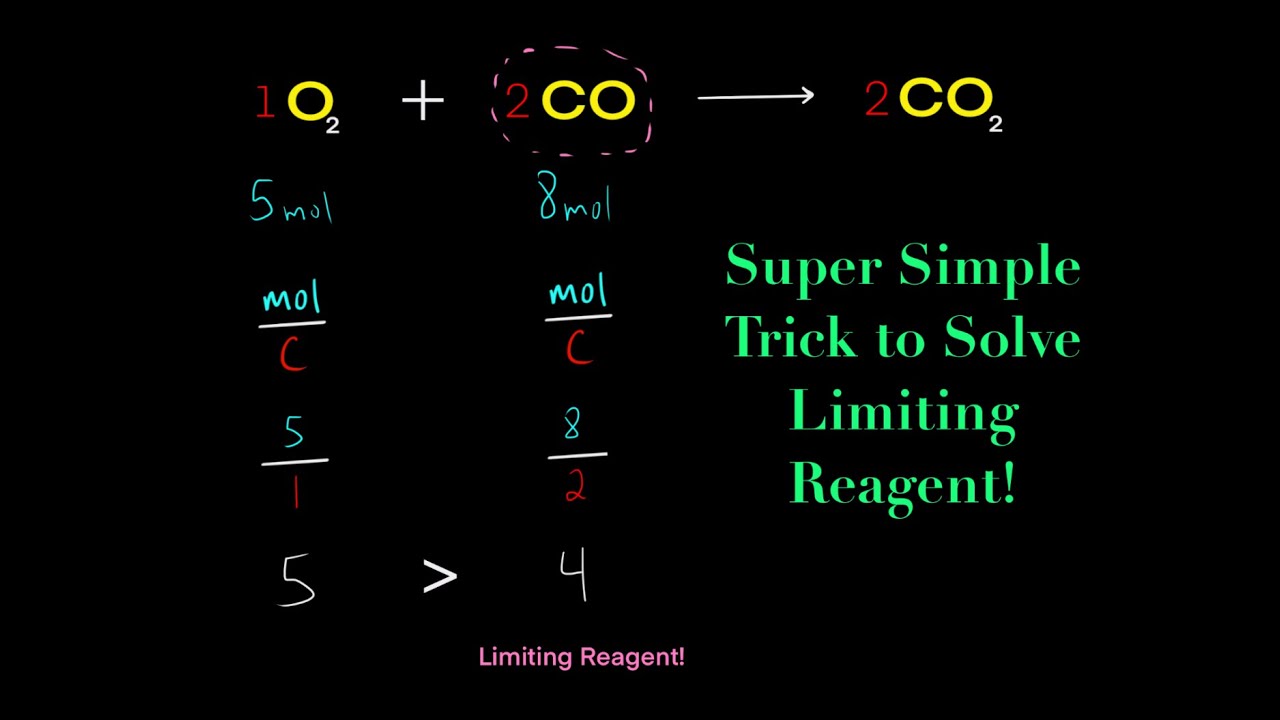
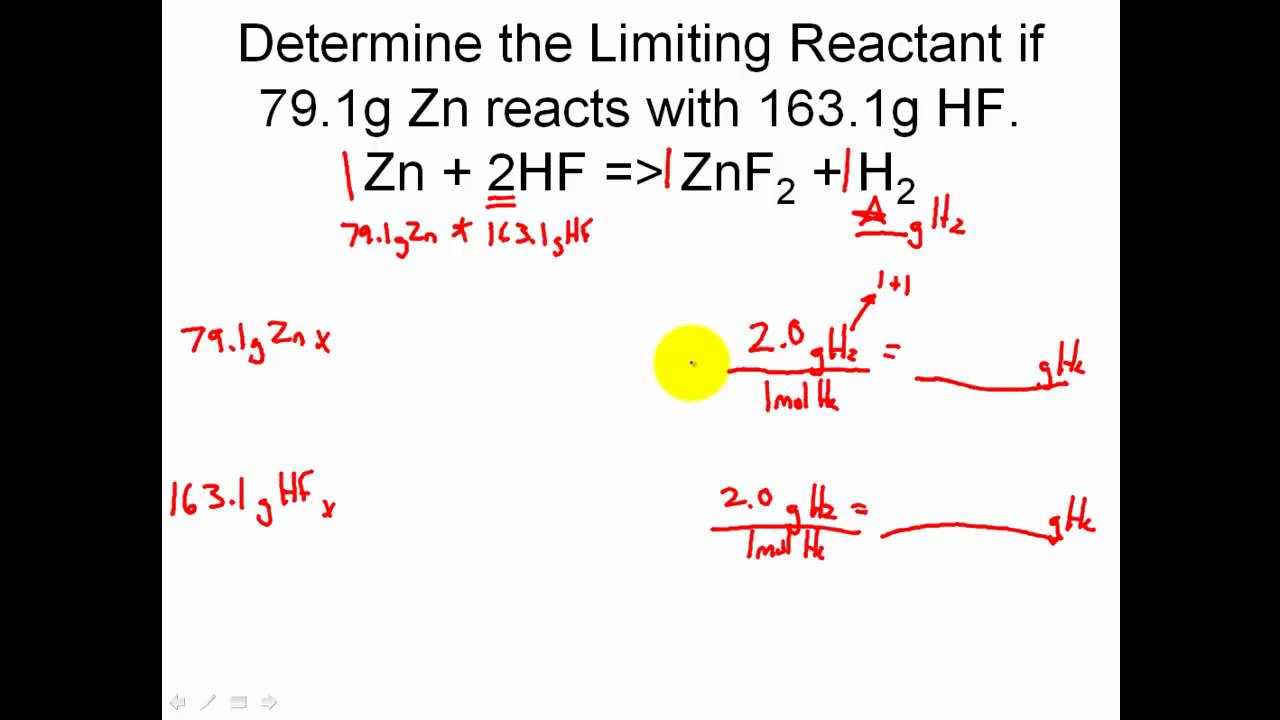
The limiting reagent is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. To identify the limiting reagent, you need to calculate the mole ratio of the reactants and compare it to the mole ratio of the balanced equation.
👉 Note: The limiting reagent is not always the reactant with the smallest amount, but rather the reactant that is completely consumed first.
Tip 2: Balance the Chemical Equation
A balanced chemical equation is essential for calculating the mole ratio of the reactants. Make sure to balance the equation before attempting to solve any limiting reagent problems.
| Reactant | Coefficient |
|---|---|
| A | 2 |
| B | 3 |
| C | 1 |
Tip 3: Calculate the Mole Ratio of the Reactants
To calculate the mole ratio of the reactants, you need to divide the number of moles of each reactant by the coefficient of that reactant in the balanced equation.
Formula: Mole Ratio = (Number of Moles) / (Coefficient)
For example, if you have 4 moles of A and the coefficient of A is 2, the mole ratio of A would be:
Mole Ratio = 4 / 2 = 2
Tip 4: Compare the Mole Ratio to the Balanced Equation
Once you have calculated the mole ratio of the reactants, compare it to the mole ratio of the balanced equation. The reactant with the smallest mole ratio is the limiting reagent.
For example, if the balanced equation is:
2A + 3B → C
And the mole ratio of the reactants is:
A: 2 B: 3
The limiting reagent would be A, since it has the smallest mole ratio.
Tip 5: Practice, Practice, Practice
The key to mastering limiting reagent stoichiometry is to practice as many problems as possible. Start with simple problems and gradually move on to more complex ones.
Some tips for practicing:
- Start with simple problems that involve only two reactants.
- Gradually move on to problems that involve multiple reactants.
- Practice problems that involve different types of reactions, such as combustion reactions and synthesis reactions.
- Use online resources, such as practice problems and quizzes, to test your knowledge.
By following these 5 tips, you can master limiting reagent stoichiometry and become proficient in solving chemical reaction problems.
To recap, the key points to remember are:
- Understand the concept of limiting reagent.
- Balance the chemical equation.
- Calculate the mole ratio of the reactants.
- Compare the mole ratio to the balanced equation.
- Practice, practice, practice.
What is the limiting reagent in a chemical reaction?
+The limiting reagent is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed.
How do I calculate the mole ratio of the reactants?
+To calculate the mole ratio of the reactants, you need to divide the number of moles of each reactant by the coefficient of that reactant in the balanced equation.
What is the importance of balancing the chemical equation?
+A balanced chemical equation is essential for calculating the mole ratio of the reactants and identifying the limiting reagent.