Mastering Lewis Structures: Worksheet 2 Solutions Revealed
Understanding the Basics of Lewis Structures
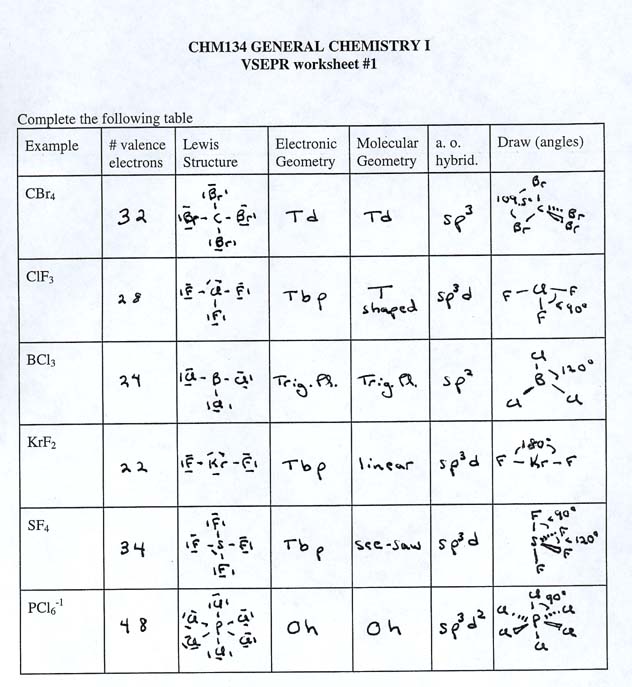
Lewis structures are a fundamental concept in chemistry that helps us visualize the arrangement of electrons in a molecule. They are a crucial tool for understanding the chemical bonding and reactivity of molecules. In this article, we will delve into the world of Lewis structures and provide solutions to Worksheet 2.
What are Lewis Structures?
Lewis structures, also known as electron dot structures, are a way of representing the valence electrons in a molecule. They are named after Gilbert N. Lewis, who first introduced this concept in 1916. Lewis structures are a two-dimensional representation of a molecule, showing the arrangement of electrons and atoms.
How to Draw Lewis Structures
Drawing Lewis structures is a step-by-step process that involves the following:
- Determine the total number of valence electrons in the molecule.
- Draw the skeleton structure of the molecule, using the atomic symbols of the atoms.
- Add electrons to the atoms, starting with the outermost energy level.
- Arrange the electrons in pairs, with each pair representing a covalent bond.
Solutions to Worksheet 2
Here are the solutions to Worksheet 2:
Problem 1: Draw the Lewis structure for CH4.
- Total valence electrons: 8 (4 from carbon and 4 from hydrogen)
- Skeleton structure: C-H-H-H-H
- Electron arrangement: H-C-H-H-H (each hydrogen atom shares one pair of electrons with carbon)
Problem 2: Draw the Lewis structure for CO2.
- Total valence electrons: 16 (4 from carbon and 12 from oxygen)
- Skeleton structure: O-C-O
- Electron arrangement: O=C=O (each oxygen atom shares two pairs of electrons with carbon)
Problem 3: Draw the Lewis structure for H2O.
- Total valence electrons: 8 (2 from hydrogen and 6 from oxygen)
- Skeleton structure: H-O-H
- Electron arrangement: H-O-H (each hydrogen atom shares one pair of electrons with oxygen)
Problem 4: Draw the Lewis structure for NH3.
- Total valence electrons: 8 (3 from nitrogen and 3 from hydrogen)
- Skeleton structure: H-N-H-H
- Electron arrangement: H-N-H-H (each hydrogen atom shares one pair of electrons with nitrogen)
Common Mistakes to Avoid
When drawing Lewis structures, there are several common mistakes to avoid:
- Incorrect electron count: Make sure to calculate the total number of valence electrons correctly.
- Incorrect skeleton structure: Ensure that the skeleton structure is correct, with the correct number of atoms and bonds.
- Incorrect electron arrangement: Double-check that the electron arrangement is correct, with each pair of electrons representing a covalent bond.
🔍 Note: Lewis structures are a simplified representation of molecules and do not show the actual shape or orientation of the molecule in space.
Conclusion
Mastering Lewis structures is a fundamental skill for any chemistry student. By understanding how to draw Lewis structures, you can visualize the arrangement of electrons in a molecule and gain insight into its chemical bonding and reactivity. Remember to avoid common mistakes and double-check your work to ensure accuracy.
What is the purpose of Lewis structures?
+Lewis structures are used to visualize the arrangement of electrons in a molecule, helping us understand chemical bonding and reactivity.
How do I determine the total number of valence electrons in a molecule?
+To determine the total number of valence electrons, add the number of valence electrons from each atom in the molecule.
What is the difference between a Lewis structure and a molecular structure?
+A Lewis structure is a two-dimensional representation of a molecule, showing the arrangement of electrons, while a molecular structure shows the actual shape and orientation of the molecule in space.