6 Ways to Master Isotope Notation
Understanding Isotope Notation: A Comprehensive Guide
Isotope notation is a crucial concept in chemistry, used to represent atoms with varying numbers of neutrons. Mastering isotope notation is essential for students and professionals alike, as it helps in understanding the properties and behavior of elements. In this article, we will explore six ways to master isotope notation, making it easier for you to grasp this fundamental concept.
1. Learn the Basics of Isotope Notation
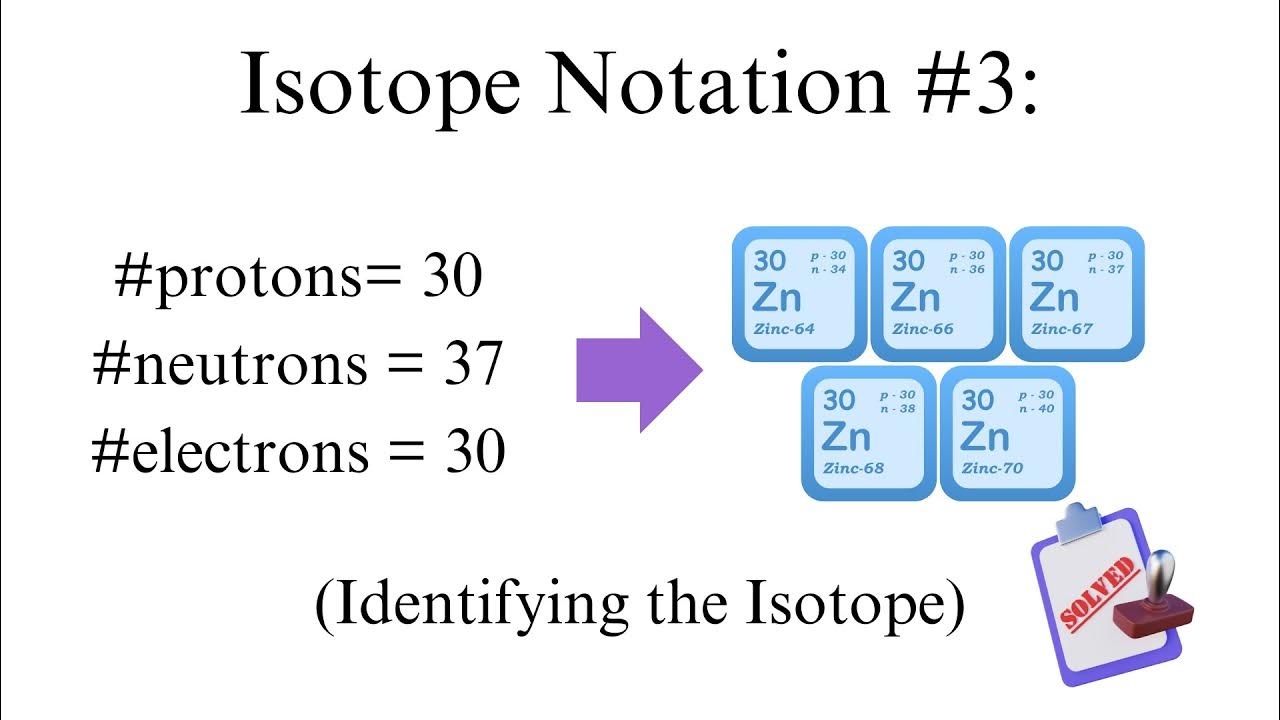
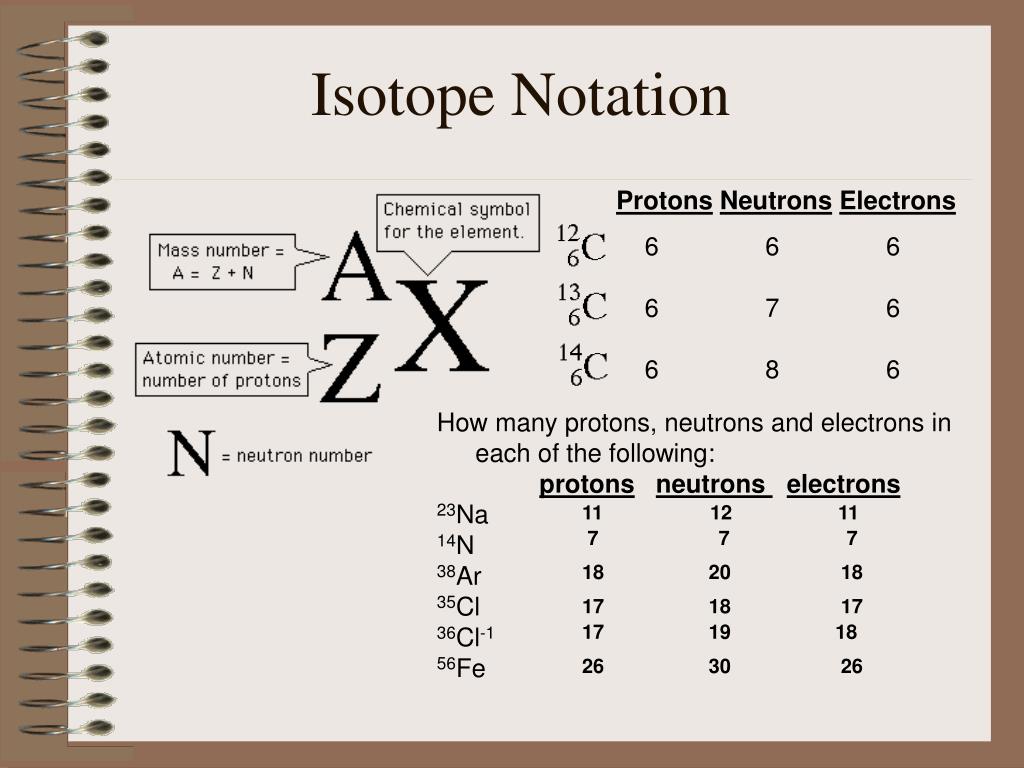
Before diving into the intricacies of isotope notation, it’s essential to understand the basics. Isotope notation is represented by the symbol of the element, followed by a superscript number indicating the mass number (the total number of protons and neutrons) and a subscript number indicating the atomic number (the number of protons). For example, carbon-14 is represented as 14C6.
2. Understand the Structure of an Atom
To fully comprehend isotope notation, you need to understand the structure of an atom. An atom consists of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons orbit around it. The number of protons determines the element of an atom, while the number of neutrons determines the isotope.
📝 Note: The atomic number (number of protons) remains the same for all isotopes of an element, while the mass number (total number of protons and neutrons) varies.
3. Practice Writing Isotope Notation
Practice is key to mastering isotope notation. Start by writing the notation for different elements, varying the number of neutrons to create different isotopes. For example, try writing the notation for carbon-12, carbon-13, and carbon-14.
4. Use Online Tools and Resources
There are numerous online tools and resources available to help you master isotope notation. Websites such as WebElements and Periodic Table offer interactive periodic tables that allow you to explore the properties of different elements and their isotopes.
5. Learn to Identify Isotopes from Their Notation
To take your understanding of isotope notation to the next level, practice identifying isotopes from their notation. For example, given the notation 238U92, you should be able to identify the element as uranium and the isotope as uranium-238.
6. Apply Isotope Notation to Real-World Scenarios
Finally, apply your knowledge of isotope notation to real-world scenarios. For example, you can explore how isotopes are used in medicine, such as the use of carbon-14 in radiocarbon dating.
| Isotope | Notation | Use |
|---|---|---|
| Carbon-14 | 14C6 | Radiocarbon dating |
| Uranium-238 | 238U92 | Nuclear power generation |
| Potassium-40 | 40K19 | Geological dating |
In conclusion, mastering isotope notation requires practice, patience, and persistence. By following these six steps, you’ll be well on your way to becoming proficient in isotope notation and enhancing your understanding of chemistry.
What is the difference between atomic number and mass number?
+The atomic number represents the number of protons in an atom’s nucleus, while the mass number represents the total number of protons and neutrons.
How are isotopes used in medicine?
+Isotopes are used in medicine for various purposes, including radiocarbon dating, cancer treatment, and medical imaging.
What is the significance of isotope notation?
+Isotope notation is essential for identifying and distinguishing between different isotopes of an element, which is crucial in various fields, including chemistry, physics, and medicine.