Mastering Isotopes and Ions: Practice Worksheet Exercises
Understanding Isotopes and Ions
Isotopes and ions are fundamental concepts in chemistry that can be challenging to grasp, but with practice and patience, you can master them. In this worksheet, we will explore exercises that will help you understand and differentiate between isotopes and ions.
What are Isotopes?
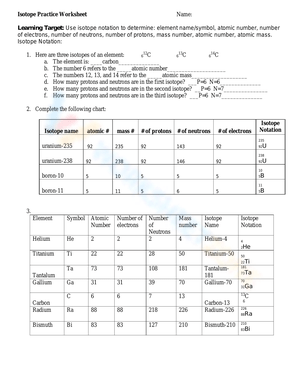
Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This means that isotopes of the same element have the same atomic number but different mass numbers.
Example:
Carbon-12, Carbon-13, and Carbon-14 are isotopes of the element carbon. They all have 6 protons in their atomic nuclei, but they differ in the number of neutrons:
- Carbon-12 has 6 protons and 6 neutrons
- Carbon-13 has 6 protons and 7 neutrons
- Carbon-14 has 6 protons and 8 neutrons
🔍 Note: Isotopes have the same chemical properties but differ in their physical properties, such as mass and radioactivity.
What are Ions?
Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. Cations are positively charged ions, while anions are negatively charged ions.
Example:
Sodium (Na) can lose an electron to form a positively charged ion, Na+.
Chlorine (Cl) can gain an electron to form a negatively charged ion, Cl-.
| Ion | Charge |
|---|---|
| Na+ | Positive |
| Cl- | Negative |
Practice Exercises
Exercise 1: Identify the isotopes in the following list:
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Nitrogen-14
- Nitrogen-15
Answer: Oxygen-16, Oxygen-17, and Oxygen-18 are isotopes of oxygen.
Exercise 2: Determine the charge on the following ions:
- Calcium (Ca)
- Oxygen (O)
- Sodium (Na)
Answer: Calcium (Ca2+), Oxygen (O2-), and Sodium (Na+)
Exercise 3: Write the symbol for the following ions:
- A positively charged ion with 11 protons and 10 electrons
- A negatively charged ion with 8 protons and 9 electrons
Answer: Na+ (sodium ion) and O- (oxygen ion)
Ion Notation
Ion notation is a way of representing ions using a symbol and a charge. The charge is indicated by a superscript number and a plus or minus sign.
Example:
Ca2+ represents a calcium ion with a charge of +2.
O2- represents an oxygen ion with a charge of -2.
Isotope Notation
Isotope notation is a way of representing isotopes using a symbol, mass number, and atomic number.
Example:
12C represents a carbon isotope with a mass number of 12 and an atomic number of 6.
14N represents a nitrogen isotope with a mass number of 14 and an atomic number of 7.
Conclusion
Mastering isotopes and ions requires practice and patience. By understanding the definitions and notation systems, you can better differentiate between these two concepts. Remember to use the correct notation and symbols when representing isotopes and ions.
What is the difference between an isotope and an ion?
+An isotope is an atom of the same element with a different number of neutrons, while an ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge.
How do you write the notation for an ion?
+Ions are represented using a symbol and a charge, indicated by a superscript number and a plus or minus sign. For example, Ca2+ represents a calcium ion with a charge of +2.
What is the purpose of isotope notation?
+Isotope notation is used to represent isotopes using a symbol, mass number, and atomic number. This notation helps to distinguish between different isotopes of the same element.
Related Terms:
- Isotope Practice Worksheet Answer Key
- Isotope Practice Worksheet answers PDF
- Isotope Practice Worksheet pdf
- Ion Practice Set Answer Key