7 Ways to Master the Ideal Gas Law
Understanding the Ideal Gas Law
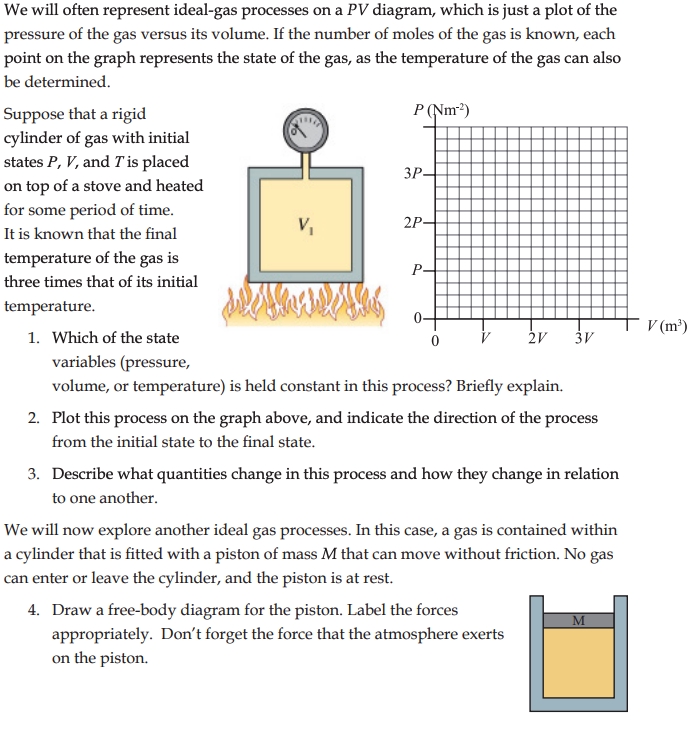
The ideal gas law is a fundamental concept in chemistry and physics that describes the behavior of gases. It is a crucial tool for understanding the properties of gases and how they interact with their surroundings. The ideal gas law is a simple equation that relates the pressure, volume, temperature, and number of moles of a gas. However, mastering the ideal gas law can be challenging, especially for students who are new to chemistry and physics.
What is the Ideal Gas Law?
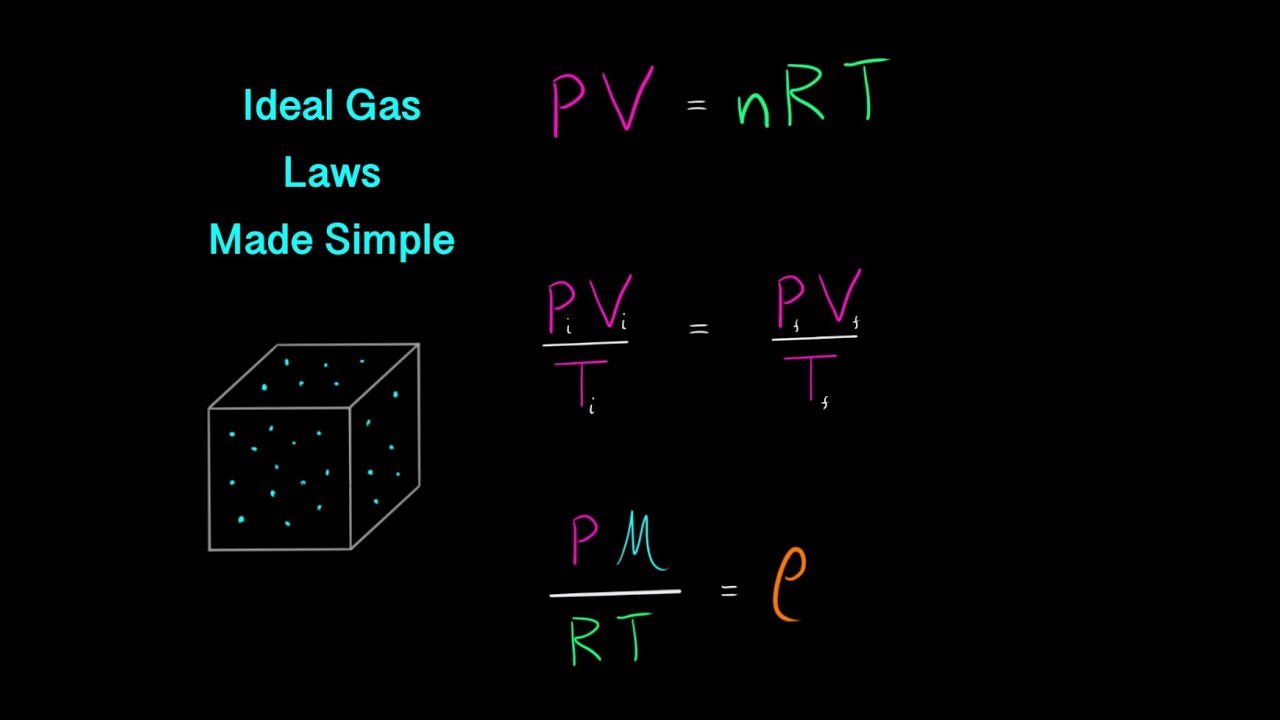
The ideal gas law is a mathematical equation that describes the behavior of ideal gases. It is expressed as:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the gas constant
- T is the temperature of the gas in Kelvin
The ideal gas law is a combination of three simpler laws:
- Boyle’s Law: P1V1 = P2V2
- Charles’ Law: V1/T1 = V2/T2
- Avogadro’s Law: V1/n1 = V2/n2
These laws were combined to form the ideal gas law, which provides a comprehensive understanding of the behavior of ideal gases.
Mastering the Ideal Gas Law
Mastering the ideal gas law requires practice, patience, and a deep understanding of the underlying concepts. Here are seven ways to help you master the ideal gas law:
1. Understand the Variables
To master the ideal gas law, you need to understand the variables involved. Make sure you know the definitions of pressure, volume, temperature, and number of moles. Understand the units of measurement for each variable and how to convert between them.
- Pressure: measured in pascals (Pa) or atmospheres (atm)
- Volume: measured in cubic meters (m³) or liters (L)
- Temperature: measured in Kelvin (K)
- Number of moles: measured in moles (mol)
2. Practice Conversions
Conversions are a crucial part of mastering the ideal gas law. Practice converting between different units of measurement for each variable. For example, convert pressure from pascals to atmospheres, or convert volume from cubic meters to liters.
3. Use the Ideal Gas Law to Solve Problems
The best way to master the ideal gas law is to practice using it to solve problems. Start with simple problems and gradually move on to more complex ones. Use online resources or textbooks to find practice problems.
| Problem | Given Values | Unknown Value |
|---|---|---|
| Find the pressure of a gas if the volume is 2L and the temperature is 300K. | V = 2L, T = 300K, n = 1mol | P |
| Find the volume of a gas if the pressure is 2atm and the temperature is 400K. | P = 2atm, T = 400K, n = 2mol | V |
4. Use Real-World Examples
Using real-world examples is a great way to master the ideal gas law. Think about situations where the ideal gas law is used in real life, such as in scuba diving, weather forecasting, or industrial processes.
- Scuba diving: The ideal gas law is used to calculate the pressure of gases in scuba tanks and to determine the safe depth for diving.
- Weather forecasting: The ideal gas law is used to predict weather patterns and to calculate the pressure and temperature of air masses.
5. Watch Video Tutorials
Video tutorials are an excellent way to visualize the ideal gas law and to understand how it works. Watch videos that explain the ideal gas law and provide examples of how to use it.
6. Join Online Communities
Joining online communities is a great way to connect with other students who are also learning about the ideal gas law. Ask questions, share resources, and get help from others who are also mastering the ideal gas law.
7. Take Practice Quizzes
Practice quizzes are an excellent way to test your knowledge of the ideal gas law. Take online quizzes or create your own quizzes to test your understanding of the ideal gas law.
🤔 Note: Mastering the ideal gas law takes time and practice. Don't be discouraged if you don't understand it at first. Keep practicing, and you will eventually master it.
In summary, mastering the ideal gas law requires a deep understanding of the underlying concepts, practice, and patience. By following these seven tips, you can master the ideal gas law and become proficient in solving problems and applying it to real-world situations.
What is the ideal gas law?
+The ideal gas law is a mathematical equation that describes the behavior of ideal gases. It is expressed as PV = nRT, where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the gas constant, and T is the temperature of the gas in Kelvin.
What are the variables involved in the ideal gas law?
+The variables involved in the ideal gas law are pressure, volume, temperature, and number of moles.
How can I master the ideal gas law?
+To master the ideal gas law, you need to understand the underlying concepts, practice using it to solve problems, and take practice quizzes. You can also watch video tutorials, join online communities, and use real-world examples to help you learn.