5 Ways to Master Graham's Law of Effusion

Understanding Graham's Law of Effusion
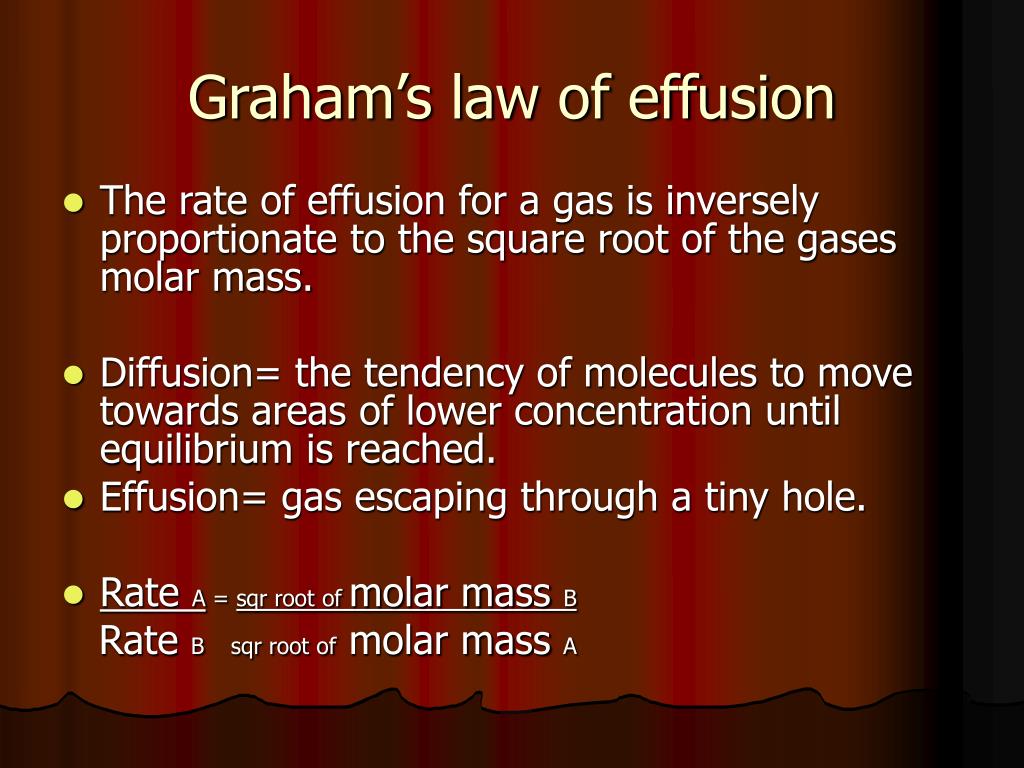
Graham’s Law of Effusion is a fundamental concept in chemistry that explains the rate at which gases diffuse through a porous material or a small opening. The law, formulated by Thomas Graham in 1848, states that the rate of effusion of a gas is inversely proportional to the square root of its molecular weight. Mastering Graham’s Law is crucial for understanding various chemical processes, including the separation of gases, the calculation of gas rates, and the analysis of gas mixtures.
What is Graham's Law of Effusion?
Graham’s Law is mathematically expressed as:
Rate of Effusion ∝ 1 / √Molecular Weight
Where:
- Rate of Effusion is the rate at which a gas diffuses through a porous material or a small opening
- Molecular Weight is the average molecular weight of the gas molecules
The law implies that lighter gases will diffuse faster than heavier gases. This concept has significant implications in various chemical applications, including the production of nitrogen and oxygen, the calculation of gas flow rates, and the analysis of gas mixtures.
5 Ways to Master Graham's Law of Effusion
1. Understand the Molecular Weight
To apply Graham’s Law, it is essential to understand the concept of molecular weight. Molecular weight is the sum of the atomic weights of the atoms in a molecule. For example, the molecular weight of oxygen (O2) is 32 g/mol, while that of nitrogen (N2) is 28 g/mol.
- Example: Calculate the molecular weight of carbon dioxide (CO2)
- Atomic weight of carbon © = 12 g/mol
- Atomic weight of oxygen (O) = 16 g/mol
- Molecular weight of CO2 = 12 + 2(16) = 44 g/mol
2. Practice Calculations
To master Graham’s Law, practice calculating the rate of effusion for different gases. Use the formula:
Rate of Effusion ∝ 1 / √Molecular Weight
- Example: Compare the rate of effusion of oxygen (O2) and nitrogen (N2)
- Molecular weight of O2 = 32 g/mol
- Molecular weight of N2 = 28 g/mol
- Rate of Effusion of O2 / Rate of Effusion of N2 = √(28⁄32) = 0.94
3. Use Analogies and Visualizations
Analogies and visualizations can help to simplify complex concepts, including Graham’s Law. Consider the following analogy:
Imagine a group of people trying to escape a crowded room. The rate at which they escape depends on their size and agility. Similarly, gas molecules escape through a porous material or a small opening at a rate that depends on their molecular weight.
4. Analyze Real-World Applications
Graham’s Law has numerous real-world applications, including the production of nitrogen and oxygen, the calculation of gas flow rates, and the analysis of gas mixtures. Analyze these applications to appreciate the significance of the law.
- Example: The production of nitrogen and oxygen
- Nitrogen and oxygen are separated from air using a process called fractional distillation
- Graham’s Law is used to calculate the rate of effusion of each gas and optimize the separation process
5. Use Online Resources and Tools
Online resources and tools can provide interactive simulations, quizzes, and exercises to help master Graham’s Law. Utilize these resources to practice calculations, analyze real-world applications, and reinforce understanding of the law.
| Resource | Description |
|---|---|
| PhET Interactive Simulations | Interactive simulations to explore Graham's Law and other chemical concepts |
| Khan Academy | Video lectures and quizzes to learn Graham's Law and other chemistry topics |
| ChemTube3D | 3D animations and interactive simulations to visualize chemical reactions and processes |
📝 Note: Mastering Graham's Law requires practice and reinforcement. Use online resources and tools to supplement learning and stay motivated.
In summary, mastering Graham’s Law of Effusion requires a combination of understanding the molecular weight, practicing calculations, using analogies and visualizations, analyzing real-world applications, and utilizing online resources and tools. By following these five ways, students and professionals can develop a deep understanding of the law and its significance in various chemical applications.