5 Ways to Master Empirical and Molecular Formulas
Unlocking the Secrets of Empirical and Molecular Formulas
Empirical and molecular formulas are the fundamental building blocks of chemistry, allowing us to understand the composition and structure of molecules. Mastering these formulas is crucial for any aspiring chemist, and with practice and dedication, anyone can become proficient in writing and interpreting them. In this article, we will explore five ways to master empirical and molecular formulas, from understanding the basics to applying them in complex chemical reactions.
1. Understanding the Basics: Empirical Formulas
An empirical formula represents the simplest whole-number ratio of atoms in a molecule. It is the most basic way to express the composition of a molecule, and it is essential to understand how to write and interpret empirical formulas. To start, let’s consider the empirical formula for glucose, C6H12O6. This formula tells us that for every six carbon atoms, there are twelve hydrogen atoms and six oxygen atoms.
- Key concept: The empirical formula is the simplest whole-number ratio of atoms in a molecule.
- Example: Glucose, C6H12O6
🔍 Note: Empirical formulas do not provide information about the arrangement of atoms in a molecule.
2. Calculating Empirical Formulas
To calculate an empirical formula, we need to know the percentage composition of the molecule. Let’s consider a molecule with the following percentage composition:

| Element | Percentage Composition |
|---|---|
| Carbon | 40.0% |
| Hydrogen | 6.7% |
| Oxygen | 53.3% |
To calculate the empirical formula, we need to assume a sample size of 100 grams and convert the percentage composition to grams.
| Element | Grams |
|---|---|
| Carbon | 40.0 g |
| Hydrogen | 6.7 g |
| Oxygen | 53.3 g |
Next, we need to convert the grams to moles using the atomic masses of each element.
| Element | Moles |
|---|---|
| Carbon | 40.0 g / 12.01 g/mol = 3.33 mol |
| Hydrogen | 6.7 g / 1.01 g/mol = 6.63 mol |
| Oxygen | 53.3 g / 16.00 g/mol = 3.33 mol |
Finally, we can divide each mole value by the smallest number of moles to get the simplest whole-number ratio.
| Element | Simplest Ratio |
|---|---|
| Carbon | 1 |
| Hydrogen | 2 |
| Oxygen | 1 |
The empirical formula for this molecule is CH2O.
- Key concept: To calculate an empirical formula, we need to know the percentage composition of the molecule and convert it to grams, moles, and finally, the simplest whole-number ratio.
- Example: CH2O
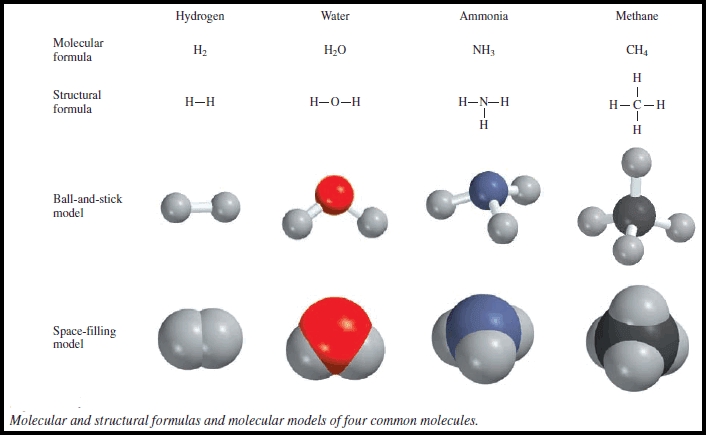
3. Understanding Molecular Formulas
A molecular formula represents the actual number of atoms in a molecule. It is more detailed than an empirical formula and provides information about the molecular structure. To write a molecular formula, we need to know the empirical formula and the molecular weight of the molecule.
- Key concept: A molecular formula represents the actual number of atoms in a molecule.
- Example: Glucose, C6H12O6 (molecular formula)
4. Converting Empirical to Molecular Formulas
To convert an empirical formula to a molecular formula, we need to know the empirical formula and the molecular weight of the molecule. Let’s consider the empirical formula CH2O and a molecular weight of 180 g/mol.
- Step 1: Calculate the empirical formula weight: CH2O = 12.01 + 2(1.01) + 16.00 = 30.04 g/mol
- Step 2: Divide the molecular weight by the empirical formula weight: 180 g/mol ÷ 30.04 g/mol = 6
- Step 3: Multiply the empirical formula by the multiplier: CH2O × 6 = C6H12O6
The molecular formula for this molecule is C6H12O6.
- Key concept: To convert an empirical formula to a molecular formula, we need to know the empirical formula, molecular weight, and multiplier.
- Example: CH2O → C6H12O6
5. Applying Empirical and Molecular Formulas in Chemical Reactions
Empirical and molecular formulas are essential tools for writing and balancing chemical equations. By understanding the composition and structure of molecules, we can predict the products of chemical reactions and balance equations. Let’s consider the combustion reaction of glucose:
C6H12O6 + O2 → CO2 + H2O
To balance this equation, we need to ensure that the number of atoms of each element is the same on both the reactant and product sides.
- Key concept: Empirical and molecular formulas are essential tools for writing and balancing chemical equations.
- Example: C6H12O6 + O2 → CO2 + H2O
In conclusion, mastering empirical and molecular formulas is a fundamental skill for any aspiring chemist. By understanding the basics, calculating empirical formulas, understanding molecular formulas, converting empirical to molecular formulas, and applying them in chemical reactions, we can gain a deeper understanding of the composition and structure of molecules.
What is the difference between empirical and molecular formulas?
+Empirical formulas represent the simplest whole-number ratio of atoms in a molecule, while molecular formulas represent the actual number of atoms in a molecule.
How do I calculate an empirical formula?
+To calculate an empirical formula, you need to know the percentage composition of the molecule, convert it to grams, moles, and finally, the simplest whole-number ratio.
What is the purpose of molecular formulas?
+Molecular formulas provide information about the molecular structure and are essential tools for writing and balancing chemical equations.