5 Ways to Master Elements Mixtures Compounds
Understanding the Basics of Elements, Mixtures, and Compounds
When it comes to chemistry, understanding the differences between elements, mixtures, and compounds is crucial. These three terms are often used interchangeably, but they have distinct meanings. In this article, we will explore the definitions of elements, mixtures, and compounds, and provide five ways to master their differences.
Elements
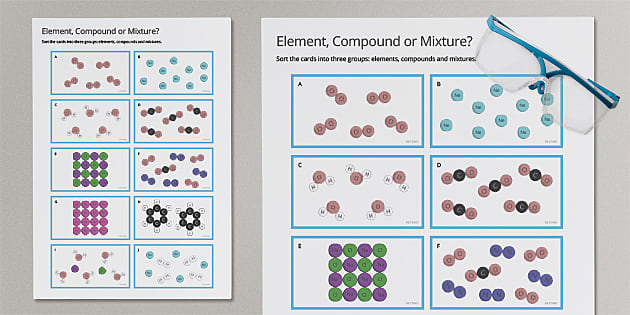
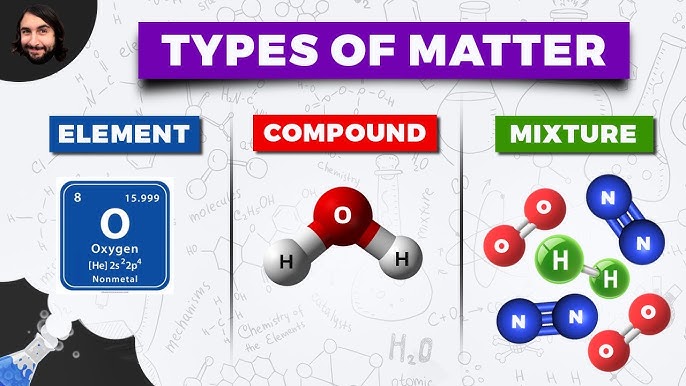
Elements are the building blocks of matter. They are substances that consist of only one type of atom and cannot be broken down into simpler substances by chemical means. Examples of elements include hydrogen, oxygen, and carbon. Elements are represented by a unique symbol, such as H for hydrogen or O for oxygen.
Mixtures
Mixtures, on the other hand, are physical combinations of two or more substances. They can be separated into their individual components by physical means, such as filtration or distillation. Examples of mixtures include air, seawater, and blood. Mixtures can be classified into two main categories: homogeneous and heterogeneous. Homogeneous mixtures have a uniform composition throughout, while heterogeneous mixtures have a non-uniform composition.
Compounds
Compounds are chemical combinations of two or more elements. They are formed when elements share electrons or exchange electrons to form a chemical bond. Compounds have properties that are different from those of their individual elements. Examples of compounds include water (H2O), carbon dioxide (CO2), and ammonia (NH3). Compounds can be classified into two main categories: molecular compounds and ionic compounds.
5 Ways to Master Elements, Mixtures, and Compounds
Now that we have understood the definitions of elements, mixtures, and compounds, let’s explore five ways to master their differences:
1. Learn the Symbols and Formulas
One of the best ways to master elements, mixtures, and compounds is to learn their symbols and formulas. Elements are represented by a unique symbol, while compounds are represented by a formula that shows the ratio of their constituent elements. For example, the symbol for hydrogen is H, while the formula for water is H2O.
- Element Symbols: Learn the symbols for common elements, such as H for hydrogen, O for oxygen, and C for carbon.
- Compound Formulas: Learn the formulas for common compounds, such as H2O for water, CO2 for carbon dioxide, and NH3 for ammonia.
2. Practice Identifying Elements, Mixtures, and Compounds
Another way to master elements, mixtures, and compounds is to practice identifying them. You can do this by reading the definitions and examples provided earlier and then trying to identify whether a given substance is an element, mixture, or compound.
- Examples: Practice identifying the following substances as elements, mixtures, or compounds: air, water, salt, sugar, and blood.
3. Learn the Properties of Elements, Mixtures, and Compounds
Elements, mixtures, and compounds have distinct properties that can be used to identify them. For example, elements have a fixed melting and boiling point, while mixtures have a variable melting and boiling point.
- Element Properties: Learn the properties of elements, such as their melting and boiling points, density, and conductivity.
- Mixture Properties: Learn the properties of mixtures, such as their variable melting and boiling points, density, and conductivity.
- Compound Properties: Learn the properties of compounds, such as their fixed melting and boiling points, density, and conductivity.
4. Understand the Differences Between Homogeneous and Heterogeneous Mixtures
Mixtures can be classified into two main categories: homogeneous and heterogeneous. Homogeneous mixtures have a uniform composition throughout, while heterogeneous mixtures have a non-uniform composition.
- Homogeneous Mixtures: Learn the properties of homogeneous mixtures, such as their uniform composition and fixed melting and boiling points.
- Heterogeneous Mixtures: Learn the properties of heterogeneous mixtures, such as their non-uniform composition and variable melting and boiling points.
5. Use Online Resources to Practice
Finally, you can use online resources to practice identifying elements, mixtures, and compounds. There are many online quizzes and games that can help you master the differences between these three terms.
- Online Quizzes: Use online quizzes to practice identifying elements, mixtures, and compounds.
- Online Games: Use online games to practice identifying elements, mixtures, and compounds in a fun and interactive way.
📝 Note: Mastering elements, mixtures, and compounds requires practice and patience. Make sure to practice identifying these substances regularly to become proficient.
| Substance | Type | Symbol/Formula |
|---|---|---|
| Hydrogen | Element | H |
| Water | Compound | H2O |
| Air | Mixture | N/A |
In conclusion, mastering elements, mixtures, and compounds requires a deep understanding of their definitions and properties. By learning the symbols and formulas, practicing identification, learning the properties, understanding the differences between homogeneous and heterogeneous mixtures, and using online resources, you can become proficient in identifying these substances.
What is the difference between an element and a compound?
+An element is a substance that consists of only one type of atom, while a compound is a substance that consists of two or more different elements that are chemically bonded together.
What is a mixture?
+A mixture is a physical combination of two or more substances that are not chemically bonded together. Mixtures can be separated into their individual components by physical means, such as filtration or distillation.
What is the difference between a homogeneous and heterogeneous mixture?
+A homogeneous mixture has a uniform composition throughout, while a heterogeneous mixture has a non-uniform composition.
Related Terms:
- Elements and compounds Worksheet PDF