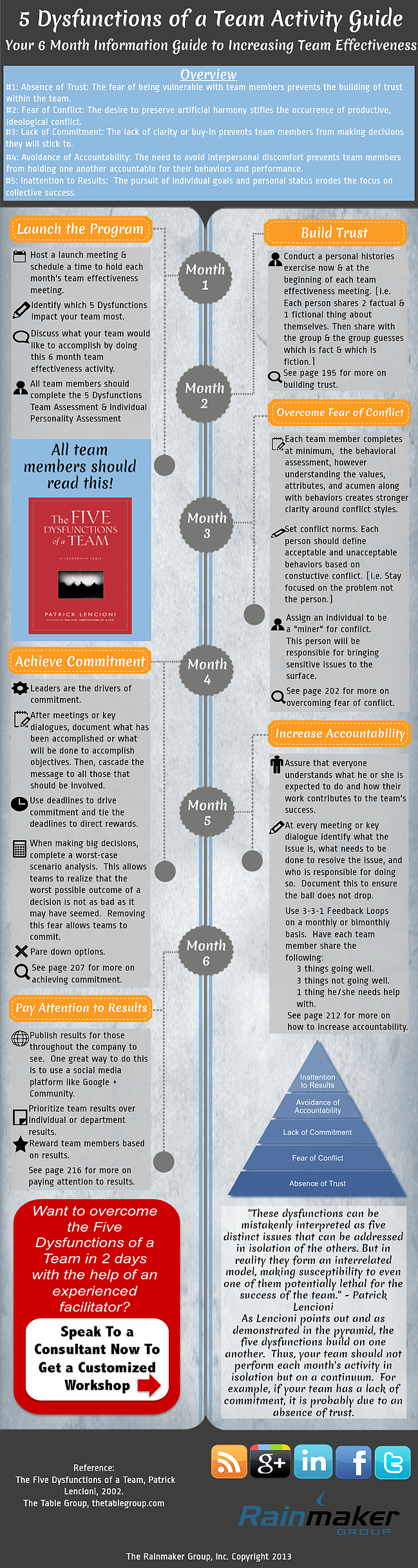
Mastering Electron Configuration Made Easy

Understanding Electron Configuration: A Comprehensive Guide
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons within an atom. It’s a crucial aspect of understanding the behavior of elements and their compounds. Mastering electron configuration can be a daunting task, but with a step-by-step approach, it can be made easy. In this article, we’ll break down the process of electron configuration, providing you with a comprehensive guide to help you grasp this essential concept.
What is Electron Configuration?
Electron configuration is the distribution of electrons within an atom’s orbitals. Orbitals are regions around the nucleus where electrons are likely to be found. The arrangement of electrons in an atom’s orbitals determines its chemical properties and reactivity. Electron configuration is typically represented by a series of numbers and letters that describe the energy level, orbital type, and number of electrons in each orbital.
Key Concepts in Electron Configuration
Before diving into the process of electron configuration, it’s essential to understand some key concepts:
- Energy levels: Energy levels, also known as electron shells, are the regions around the nucleus where electrons are found. Energy levels are designated by integers (1, 2, 3, etc.).
- Orbitals: Orbitals are the specific regions within an energy level where electrons are likely to be found. Orbitals are designated by letters (s, p, d, f).
- Electron spin: Electron spin refers to the intrinsic angular momentum of an electron. Electrons can have either a +1⁄2 or -1⁄2 spin.
Step-by-Step Guide to Electron Configuration
Now that we’ve covered the key concepts, let’s move on to the step-by-step process of electron configuration:
- Determine the number of electrons: The first step in electron configuration is to determine the number of electrons in the atom. This can be done by finding the atomic number of the element on the periodic table.
- Identify the energy levels: Next, identify the energy levels that are occupied by electrons. The energy levels are designated by integers (1, 2, 3, etc.).
- Determine the orbital types: Within each energy level, determine the orbital types (s, p, d, f) that are occupied by electrons.
- Apply the Aufbau principle: The Aufbau principle states that electrons occupy the lowest available energy levels. Apply this principle to determine the arrangement of electrons in each orbital.
- Apply the Pauli exclusion principle: The Pauli exclusion principle states that no two electrons can have the same set of quantum numbers. Apply this principle to determine the arrangement of electrons in each orbital.
- Apply Hund’s rule: Hund’s rule states that when filling orbitals of equal energy, electrons occupy each orbital singly before pairing up.
📝 Note: When applying the Aufbau principle, Pauli exclusion principle, and Hund's rule, it's essential to remember that electrons occupy the lowest available energy levels and that no two electrons can have the same set of quantum numbers.
Electron Configuration Notation
Electron configuration notation is a shorthand way of representing the arrangement of electrons in an atom. The notation consists of a series of numbers and letters that describe the energy level, orbital type, and number of electrons in each orbital. The notation is typically written in the following format:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰
In this notation:
- 1s²: The “1” represents the first energy level, “s” represents the orbital type, and “²” represents the number of electrons in the orbital.
- 2s²: The “2” represents the second energy level, “s” represents the orbital type, and “²” represents the number of electrons in the orbital.
Examples of Electron Configuration
Let’s consider a few examples of electron configuration:
- Hydrogen (H): 1s¹
- Helium (He): 1s²
- Lithium (Li): 1s² 2s¹
- Carbon ©: 1s² 2s² 2p⁶
📝 Note: Electron configuration notation can be written in different formats, but the above examples represent the most common notation used in chemistry.
Importance of Electron Configuration
Electron configuration is crucial in understanding the chemical properties and reactivity of elements. It helps us predict:
- Chemical reactivity: Electron configuration determines the chemical reactivity of an element. Elements with similar electron configurations tend to exhibit similar chemical properties.
- Physical properties: Electron configuration affects the physical properties of an element, such as its melting and boiling points.
- Compound formation: Electron configuration determines the types of compounds an element can form.
Conclusion
Mastering electron configuration requires a step-by-step approach and a thorough understanding of the key concepts involved. By following the Aufbau principle, Pauli exclusion principle, and Hund’s rule, you can determine the arrangement of electrons in an atom. Electron configuration notation provides a shorthand way of representing the arrangement of electrons, and understanding its importance is crucial in predicting the chemical properties and reactivity of elements.
What is electron configuration?
+Electron configuration is the distribution of electrons within an atom’s orbitals.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels.
What is Hund’s rule?
+Hund’s rule states that when filling orbitals of equal energy, electrons occupy each orbital singly before pairing up.