5 Tips to Ace Drawing Atoms Worksheet
Understanding the Basics of Drawing Atoms
Drawing atoms is a fundamental concept in chemistry, and it can be a bit challenging for students to grasp at first. However, with practice and patience, anyone can master the skill. A drawing atoms worksheet is a great tool to help students learn and practice drawing atoms. In this post, we will provide five tips to help students ace their drawing atoms worksheet.
Tip 1: Understand the Structure of an Atom
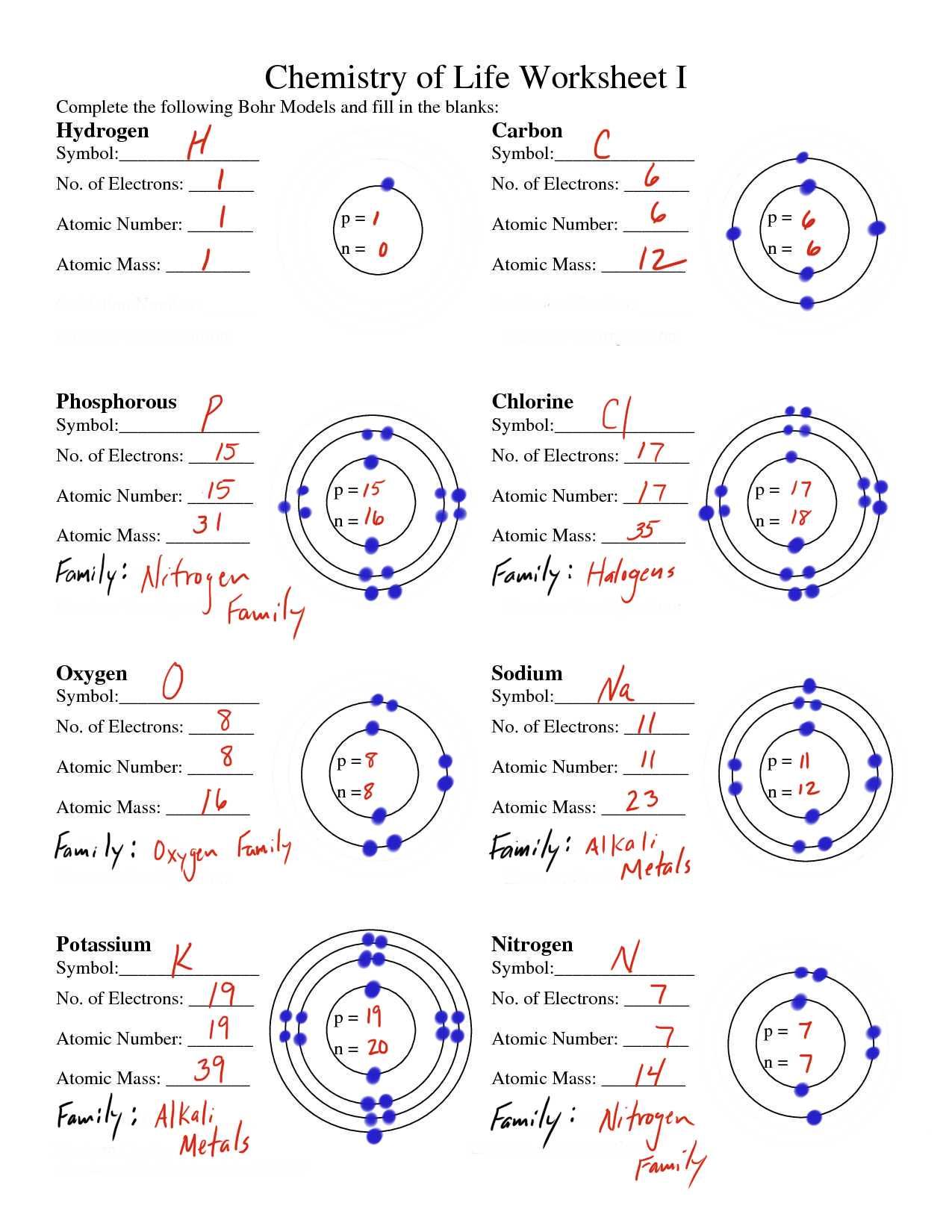
Before drawing an atom, it’s essential to understand its structure. An atom consists of three main parts: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons orbit around the nucleus. The number of protons in an atom determines the element of an atom, and the number of neutrons can vary, leading to different isotopes of the same element.
📝 Note: Understanding the structure of an atom is crucial to drawing it correctly. Make sure to review the basics before starting your worksheet.
Tip 2: Use the Correct Symbols and Notations
When drawing atoms, it’s essential to use the correct symbols and notations. The nucleus is represented by a small circle, and the electrons are represented by dots or arrows. The number of protons (atomic number) is written at the top left of the symbol, and the number of neutrons (mass number) is written at the top right. For example, the symbol for carbon-14 would be ¹⁴C₆.
📝 Note: Pay attention to the notation system used in your worksheet or textbook. Different notation systems may be used, but the concept remains the same.
Tip 3: Draw the Electron Shells Correctly
Electron shells are the regions around the nucleus where electrons orbit. The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, and the third shell can hold up to 18 electrons. When drawing electron shells, make sure to follow these rules:
- The first shell is represented by a single dot or arrow closest to the nucleus.
- The second shell is represented by two dots or arrows farther away from the nucleus.
- The third shell is represented by three dots or arrows even farther away from the nucleus.
📝 Note: Make sure to leave enough space between the nucleus and the electron shells to avoid clutter.
Tip 4: Use the Correct Number of Electrons
The number of electrons in an atom is equal to the number of protons. When drawing an atom, make sure to use the correct number of electrons. For example, if the atomic number is 6 (carbon), you should draw 6 electrons.
📝 Note: Remember that the number of electrons can vary in ions, but for neutral atoms, the number of electrons equals the number of protons.
Tip 5: Practice, Practice, Practice
Practice is key to mastering the skill of drawing atoms. The more you practice, the more comfortable you’ll become with the notation system, electron shells, and correct number of electrons.
📝 Note: Start with simple atoms and gradually move on to more complex ones. You can also find online resources or worksheets to practice drawing atoms.
What is the most important thing to remember when drawing atoms?
+Understanding the structure of an atom is crucial to drawing it correctly. Make sure to review the basics before starting your worksheet.
How do I represent the nucleus in an atom?
+The nucleus is represented by a small circle, and the number of protons (atomic number) is written at the top left of the symbol.
What is the correct notation system to use when drawing atoms?
+Pay attention to the notation system used in your worksheet or textbook. Different notation systems may be used, but the concept remains the same.
In summary, drawing atoms requires a solid understanding of the structure of an atom, correct notation system, and practice. By following these five tips, students can ace their drawing atoms worksheet and develop a strong foundation in chemistry.